Page 41 - Read Online
P. 41
Almeida et al. Vessel Plus 2021;5:44 https://dx.doi.org/10.20517/2574-1209.2021.66 Page 5 of 14
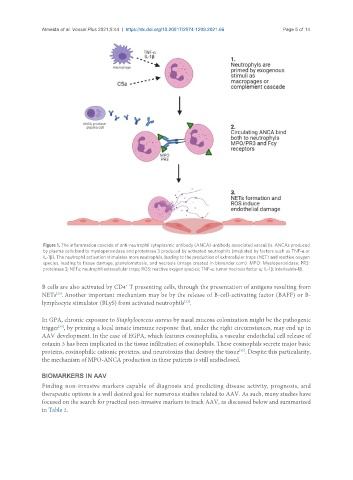
Figure 1. The inflammation cascade of anti-neutrophil cytoplasmic antibody (ANCA)-antibody associated vasculitis. ANCAs produced
by plasma cells bind to myeloperoxidase and proteinase 3 produced by activated neutrophils (mediated by factors such as TNF-α or
IL-1β). The neutrophil activation stimulates more neutrophils, leading to the production of extracellular traps (NET) and reactive oxygen
species, leading to tissue damage, granulomatosis, and necrosis (image created in biorender.com). MPO: Myeloperoxidase; PR3:
proteinase 3; NETs: neutrophil extracellular traps; ROS: reactive oxygen species; TNF-α: tumor necrosis factor α; IL-1β: interleukin-1β.
B cells are also activated by CD4 T presenting cells, through the presentation of antigens resulting from
+
[33]
NETs . Another important mechanism may be by the release of B-cell-activating factor (BAFF) or B-
lymphocyte stimulator (BLyS) from activated neutrophils .
[33]
In GPA, chronic exposure to Staphylococcus aureus by nasal mucosa colonization might be the pathogenic
trigger , by priming a local innate immune response that, under the right circumstances, may end up in
[34]
AAV development. In the case of EGPA, which features eosinophilia, a vascular endothelial cell release of
eotaxin 3 has been implicated in the tissue infiltration of eosinophils. These eosinophils secrete major basic
proteins, eosinophilic cationic proteins, and neurotoxins that destroy the tissue . Despite this particularity,
[35]
the mechanism of MPO-ANCA production in these patients is still undisclosed.
BIOMARKERS IN AAV
Finding non-invasive markers capable of diagnosis and predicting disease activity, prognosis, and
therapeutic options is a well desired goal for numerous studies related to AAV. As such, many studies have
focused on the search for practical non-invasive markers to track AAV, as discussed below and summarized
in Table 2.