Page 26 - Read Online
P. 26
Page 4 of 14 Heng et al. Vessel Plus 2023;7:31 https://dx.doi.org/10.20517/2574-1209.2023.97
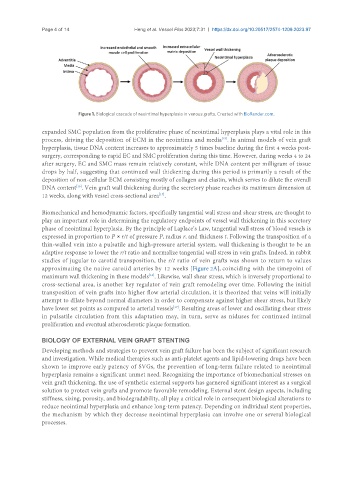
Figure 1. Biological cascade of neointimal hyperplasia in venous grafts. Created with BioRender.com.
expanded SMC population from the proliferative phase of neointimal hyperplasia plays a vital role in this
[15]
process, driving the deposition of ECM in the neointima and media . In animal models of vein graft
hyperplasia, tissue DNA content increases to approximately 5 times baseline during the first 4 weeks post-
surgery, corresponding to rapid EC and SMC proliferation during this time. However, during weeks 4 to 24
after surgery, EC and SMC mass remain relatively constant, while DNA content per milligram of tissue
drops by half, suggesting that continued wall thickening during this period is primarily a result of the
deposition of non-cellular ECM consisting mostly of collagen and elastin, which serves to dilute the overall
DNA content . Vein graft wall thickening during the secretory phase reaches its maximum dimension at
[16]
[17]
12 weeks, along with vessel cross-sectional area .
Biomechanical and hemodynamic factors, specifically tangential wall stress and shear stress, are thought to
play an important role in determining the regulatory endpoints of vessel wall thickening in this secretory
phase of neointimal hyperplasia. By the principle of Laplace’s Law, tangential wall stress of blood vessels is
expressed in proportion to P × r/t of pressure P, radius r, and thickness t. Following the transposition of a
thin-walled vein into a pulsatile and high-pressure arterial system, wall thickening is thought to be an
adaptive response to lower the r/t ratio and normalize tangential wall stress in vein grafts. Indeed, in rabbit
studies of jugular to carotid transposition, the r/t ratio of vein grafts was shown to return to values
approximating the native carotid arteries by 12 weeks [Figure 2A], coinciding with the timepoint of
[16]
maximum wall thickening in these models . Likewise, wall shear stress, which is inversely proportional to
cross-sectional area, is another key regulator of vein graft remodeling over time. Following the initial
transposition of vein grafts into higher flow arterial circulation, it is theorized that veins will initially
attempt to dilate beyond normal diameters in order to compensate against higher shear stress, but likely
have lower set points as compared to arterial vessels . Resulting areas of lower and oscillating shear stress
[17]
in pulsatile circulation from this adaptation may, in turn, serve as niduses for continued intimal
proliferation and eventual atherosclerotic plaque formation.
BIOLOGY OF EXTERNAL VEIN GRAFT STENTING
Developing methods and strategies to prevent vein graft failure has been the subject of significant research
and investigation. While medical therapies such as anti-platelet agents and lipid-lowering drugs have been
shown to improve early patency of SVGs, the prevention of long-term failure related to neointimal
hyperplasia remains a significant unmet need. Recognizing the importance of biomechanical stresses on
vein graft thickening, the use of synthetic external supports has garnered significant interest as a surgical
solution to protect vein grafts and promote favorable remodeling. External stent design aspects, including
stiffness, sizing, porosity, and biodegradability, all play a critical role in consequent biological alterations to
reduce neointimal hyperplasia and enhance long-term patency. Depending on individual stent properties,
the mechanism by which they decrease neointimal hyperplasia can involve one or several biological
processes.