Page 178 - Read Online
P. 178
Page 12 of 21 Bradshaw et al. Vessel Plus 2023;7:35 https://dx.doi.org/10.20517/2574-1209.2023.121
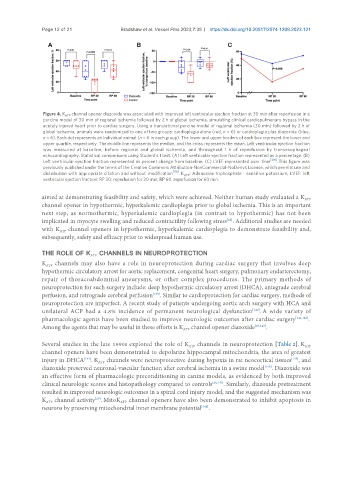
Figure 4. K channel opener diazoxide was associated with improved left ventricular ejection fraction at 30 min after reperfusion in a
ATP
porcine model of 30 min of regional ischemia followed by 2 h of global ischemia, simulating clinical cardiopulmonary bypass in the
acutely injured heart prior to cardiac surgery. Using a translational porcine model of regional ischemia (30 min) followed by 2 h of
global ischemia, animals were randomized to one of two groups: cardioplegia alone (red, n = 6) or cardioplegia plus diazoxide (blue,
n = 6). Each dot represents an individual animal (n = 6 in each group). The lower and upper borders of each box represent the lower and
upper quartile, respectively. The middle line represents the median, and the cross represents the mean. Left ventricular ejection fraction
was measured at baseline, before regional and global ischemia, and throughout 1 h of reperfusion by transesophageal
echocardiography. Statistical comparisons using Student’s t test. (A) Left ventricular ejection fraction represented as a percentage. (B)
[136]
Left ventricular ejection fraction represented as percent change from baseline. (C) LVEF represented over time . This figure was
previously published under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and
[136]
distribution with appropriate citation and without modification . K : Adenosine triphosphate - sensitive potassium; LVEF: left
ATP
ventricular ejection fraction; RP 30: reperfusion for 30 min; RP 60: reperfusion for 60 min.
aimed at demonstrating feasibility and safety, which were achieved. Neither human study evaluated a K
ATP
channel opener in hypothermic, hyperkalemic cardioplegia prior to global ischemia. This is an important
next step, as normothermic, hyperkalemic cardioplegia (in contrast to hypothermic) has not been
implicated in myocyte swelling and reduced contractility following stress . Additional studies are needed
[82]
with K channel openers in hypothermic, hyperkalemic cardioplegia to demonstrate feasibility and,
ATP
subsequently, safety and efficacy prior to widespread human use.
THE ROLE OF K CHANNELS IN NEUROPROTECTION
ATP
K channels may also have a role in neuroprotection during cardiac surgery that involves deep
ATP
hypothermic circulatory arrest for aortic replacement, congenital heart surgery, pulmonary endarterectomy,
repair of thoracoabdominal aneurysms, or other complex procedures. The primary methods of
neuroprotection for such surgery include: deep hypothermic circulatory arrest (DHCA), antegrade cerebral
perfusion, and retrograde cerebral perfusion . Similar to cardioprotection for cardiac surgery, methods of
[139]
neuroprotection are imperfect. A recent study of patients undergoing aortic arch surgery with HCA and
unilateral ACP had a 4.8% incidence of permanent neurological dysfunction . A wide variety of
[140]
pharmacologic agents have been studied to improve neurologic outcomes after cardiac surgery [141,142] .
Among the agents that may be useful in these efforts is K channel opener diazoxide [27,143] .
ATP
Several studies in the late 1990s explored the role of K channels in neuroprotection [Table 2]. K
ATP
ATP
channel openers have been demonstrated to depolarize hippocampal mitochondria, the area of greatest
injury in DHCA . K channels were neuroprotective during hypoxia in rat neocortical tissues , and
[145]
[144]
ATP
diazoxide preserved neuronal-vascular function after cerebral ischemia in a swine model . Diazoxide was
[146]
an effective form of pharmacologic preconditioning in canine models, as evidenced by both improved
clinical neurologic scores and histopathology compared to controls [28,147] . Similarly, diazoxide pretreatment
resulted in improved neurologic outcomes in a spinal cord injury model, and the suggested mechanism was
[29]
K channel activity . MitoK channel openers have also been demonstrated to inhibit apoptosis in
ATP
ATP
neurons by preserving mitochondrial inner membrane potential .
[148]