Page 25 - Read Online
P. 25
Page 8 of 19 Ji et al. Rare Dis Orphan Drugs J 2023;2:26 https://dx.doi.org/10.20517/rdodj.2023.30
[1]
Comparisons between Australian and Californian NBS programs have been made . The way conditions are listed and named differs and affects
how they are counted, confounding comparisons. HGSA defines Category 1 “target” conditions as those formally approved for screening in
Australia and Category 2 “incidental” findings as conditions that have the same marker metabolite as a target condition. RUSP is a list of disorders
that the United States Secretary of the Department of Health and Human Services recommends for states to screen as part of their state universal
NBS programs. The RUSP defines “core conditions” as conditions for which screening should be mandated. “Secondary conditions”, as defined by
the RUSP, are additional conditions identified because they are part of the differential diagnosis of a condition in the core panel. SCADD, OTC, and
CPS deficiency, which are screened for in California, have been specifically removed from Australian NBS for reasons noted. *Has been referred to
the Australian MSAC health technology assessment process but is not currently screened in Australia. **Technical advice sought. Other
conditions for which technical advice is being sought include Batten disease, Fabry disease, Gaucher disease, Krabbe disease, and Niemann-Pick
#
disease types A & B. X linked Adrenoleukodystrophy recommended for inclusion in Australian NBS-state and territory governments to decide
regarding implementation. This table is current, to the best of our knowledge, as of 23 October 2023. RUSP: recommended universal screening
panel; NBS: newborn bloodspot screening; HGSA: Human Genetics Society of Australasia; SCADD: short-chain acyl-CoA dehydrogenase
deficiency; OTC: ornithine transcarbamylase deficiency; CPS deficiency: carbamoyl phosphate synthetase deficiency; MSAC: Medical Services
Advisory Committee.
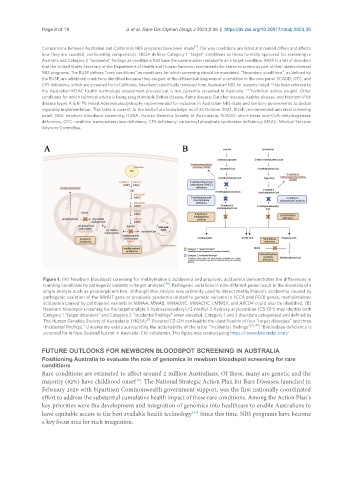
Figure 1. (A) Newborn bloodspot screening for methylmalonic acidaemia and propionic acidaemia demonstrates the differences in
counting conditions by pathogenic variants or target analytes [36] . Pathogenic variations in nine different genes result in the elevation of a
single analyte such as propionylcarnitine. Although this analyte was primarily used to detect methylmalonic acidaemia caused by
pathogenic variation of the MMUT gene or propionic academia related to genetic variants in PCCA and PCCB genes, methylmalonic
acidaemia caused by pathogenic variants in MMAA, MMAB, MMADHC, MMACHC, LMBRD1, and ABCD4 could also be identified; (B)
Newborn bloodspot screening for the target analyte 3-hydroxyisovaleryl-/2-methyl-3-hydroxy acylcarnitine (C5-OH) may identify both
Category 1 “target disorders” and Category 2 “incidental findings” when elevated. Category 1 and 2 disorders categorised and defined by
[1]
The Human Genetics Society of Australasia (HGSA) . Elevated C5-OH can lead to the identification of four “target disorders” and three
“incidental findings.” Uncertainty exists surrounding the actionability of the latter “incidental findings” [37,38] . *Biotinidase deficiency is
screened for in New Zealand but not in Australia. Cbl: cobalamin. This figure was created using https://www.biorender.com/.
FUTURE OUTLOOKS FOR NEWBORN BLOODSPOT SCREENING IN AUSTRALIA
Positioning Australia to evaluate the role of genomics in newborn bloodspot screening for rare
conditions
Rare conditions are estimated to affect around 2 million Australians. Of these, many are genetic and the
[48]
majority (82%) have childhood onset . The National Strategic Action Plan for Rare Diseases, launched in
February 2020 with bipartisan Commonwealth government support, was the first nationally coordinated
effort to address the substantial cumulative health impact of these rare conditions. Among the Action Plan’s
key priorities were the development and integration of genomics into healthcare to enable Australians to
[49]
have equitable access to the best available health technology . Since this time, NBS programs have become
a key focus area for such integration.