Page 23 - Read Online
P. 23
Page 6 of 19 Ji et al. Rare Dis Orphan Drugs J 2023;2:26 https://dx.doi.org/10.20517/rdodj.2023.30
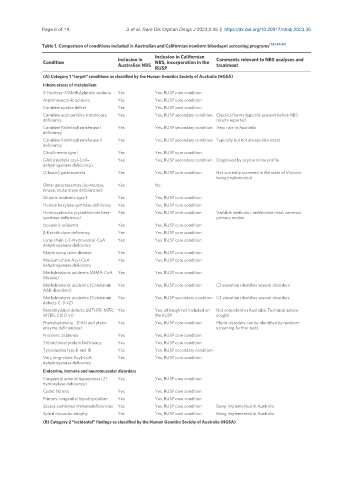
Table 1. Comparison of conditions included in Australian and Californian newborn bloodspot screening programs [1,37,40-43]
Inclusion in Californian
Inclusion in Comments relevant to NBS analyses and
Condition NBS, incorporation in the
Australian NBS RUSP treatment
(A) Category 1 “target” conditions as classified by the Human Genetics Society of Australia (HGSA)
Inborn errors of metabolism
3-Hydroxy-3-Methylglutaric aciduria Yes Yes, RUSP core condition
Argininosuccinic aciduria Yes Yes, RUSP core condition
Carnitine uptake defect Yes Yes, RUSP core condition
Carnitine acylcarnitine translocase Yes Yes, RUSP secondary condition Classical forms typically present before NBS
deficiency results reported
Carnitine Palmitoyltransferase I Yes Yes, RUSP secondary condition Very rare in Australia
deficiency
Carnitine Palmitoyltransferase II Yes Yes, RUSP secondary condition Typically but not always late onset
deficiency
Citrullinemia type I Yes Yes, RUSP core condition
GAII (multiple acyl-CoA- Yes Yes, RUSP secondary condition Diagnosed by acylcarnitine profile
dehydrogenase deficiency)
(Classic) galactosemia Yes Yes, RUSP core condition Not currently screened in the state of Victoria-
being implemented
Other galactosemias (epimerase, Yes No
kinase, mutarotase deficiencies)
Glutaric acidemia type I Yes Yes, RUSP core condition
Holocarboxylase synthase deficiency Yes Yes, RUSP core condition
Homocystinuria (cystathionine beta- Yes Yes, RUSP core condition Variable methods - methionine most common
synthase deficiency) primary marker
Isovaleric acidemia Yes Yes, RUSP core condition
β-Ketothiolase deficiency Yes Yes, RUSP core condition
Long-chain L-3-Hydroxyacyl-CoA Yes Yes, RUSP core condition
dehydrogenase deficiency
Maple syrup urine disease Yes Yes, RUSP core condition
Medium-chain Acyl-CoA Yes Yes, RUSP core condition
dehydrogenase deficiency
Methylmalonic acidemia (MMA-CoA Yes Yes, RUSP core condition
Mutase)
Methylmalonic acidemia (Cobalamin Yes Yes, RUSP core condition C3 elevation identifies several disorders
A&B disorders)
Methylmalonic acidemia (Cobalamin Yes Yes, RUSP secondary condition C3 elevation identifies several disorders
defects C, D v2)
Remethylation defects (MTHFR, MTR, Yes Yes, although not included on Not consistent in Australia. Technical advice
MTRR, Cbl D v1) the RUSP sought
Phenylketonuria - (PAH and pterin Yes Yes, RUSP core condition Pterin disorders can be identified by newborn
enzyme deficiencies) screening-further tests
Propionic acidemia Yes Yes, RUSP core condition
Trifunctional protein Deficiency Yes Yes, RUSP core condition
Tyrosinemia type II and III Yes Yes, RUSP secondary condition
Very long-chain Acyl-CoA Yes Yes, RUSP core condition
dehydrogenase deficiency
Endocrine, immune and neuromuscular disorders
Congenital adrenal hyperplasia (21- Yes Yes, RUSP core condition
hydroxylase deficiency)
Cystic fibrosis Yes Yes, RUSP core condition
Primary congenital hypothyroidism Yes Yes, RUSP core condition
Severe combined immunodeficiencies Yes Yes, RUSP core condition Being implemented in Australia
Spinal muscular atrophy Yes Yes, RUSP core condition Being implemented in Australia
(B) Category 2 “incidental” findings as classified by the Human Genetics Society of Australia (HGSA)