Page 9 - Read Online
P. 9
Chen et al. Rare Dis Orphan Drugs J 2022;1:15 https://dx.doi.org/10.20517/rdodj.2022.18 Page 5 of 12
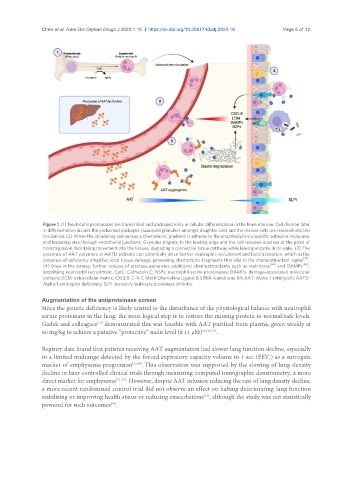
Figure 1. (1) Neutrophil proteinases are transcribed and packaged early in cellular differentiation in the bone marrow. Cell division later
in differentiation divides the preformed packages (azurophil granules) amongst daughter cells and the mature cells are released into the
circulation. (2) When the circulating cell senses a chemotactic gradient, it adheres to the endothelium via specific adhesion molecules
and transmigrates through endothelial junctions. Granules migrate to the leading edge and the cell releases elastase at the point of
transmigration, facilitating movement into the tissues, degrading a connective tissue pathway while leaving enzyme in its wake. (3) The
presence of AAT polymers in AATD patients can potentially drive further neutrophil recruitment and local activation, which in the
[31]
presence of deficiency amplifies local tissue damage, generating chemotactic fragments that add to the chemoattractant signal .
[44] [45]
(4) Once in the airway, further release of elastase generates additional chemoattractants such as matrikines and DAMPs ,
amplifying neutrophil recruitment. CatC: Cathepsin C; NSPs: neutrophil serine proteinases; DAMPs: damage-associated molecular
patterns; ECM: extracellular matrix; CXCL8: C-X-C Motif Chemokine Ligand 8; LTB4: leukotriene B4; AAT: Alpha-1 antitrypsin; AATD:
Alpha-1 antitrypsin deficiency; SLPI: secretory leukocyte proteinase inhibitor.
Augmentation of the antiproteinase screen
Since the genetic deficiency is likely central to the disturbance of the physiological balance with neutrophil
serine proteinase in the lung, the most logical step is to restore the missing protein to normal/safe levels.
[10]
Gadek and colleagues demonstrated this was feasible with AAT purified from plasma, given weekly at
60 mg/kg to achieve a putative “protective” nadir level (≥ 11 µM) [24,46,47] .
Registry data found that patients receiving AAT augmentation had slower lung function decline, especially
in a limited midrange detected by the forced expiratory capacity volume in 1 sec (FEV ) as a surrogate
1
marker of emphysema progression [13,48] . This observation was supported by the slowing of lung density
decline in later controlled clinical trials through measuring computed tomographic densitometry, a more
direct marker for emphysema [15-17] . However, despite AAT infusion reducing the rate of lung density decline,
a more recent randomised control trial did not observe an effect on halting deteriorating lung function
stabilising or improving health status or reducing exacerbations , although the study was not statistically
[18]
powered for such outcomes .
[49]