Page 31 - Read Online
P. 31
Mazur et al. Rare Dis Orphan Drugs J 2023;2:1 https://dx.doi.org/10.20517/rdodj.2022.12 Page 5 of 10
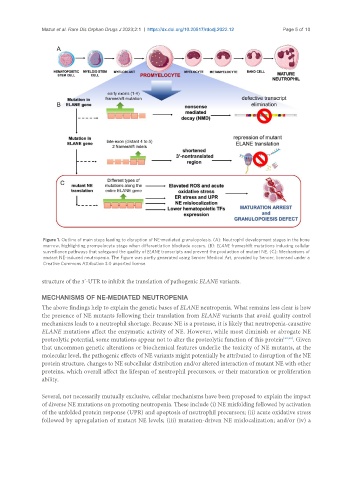
Figure 1. Outline of main steps leading to disruption of NE-mediated granulopoiesis. (A): Neutrophil development stages in the bone
marrow, highlighting promyelocyte stage when differentiation blockade occurs. (B): ELANE frameshift mutations inducing cellular
surveillance pathways that safeguard the quality of ELANE transcripts and prevent the production of mutant NE. (C): Mechanisms of
mutant NE-induced neutropenia. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a
Creative Commons Attribution 3.0 unported license.
structure of the 3’-UTR to inhibit the translation of pathogenic ELANE variants.
MECHANISMS OF NE-MEDIATED NEUTROPENIA
The above findings help to explain the genetic bases of ELANE neutropenia. What remains less clear is how
the presence of NE mutants following their translation from ELANE variants that avoid quality control
mechanisms leads to a neutrophil shortage. Because NE is a protease, it is likely that neutropenia-causative
ELANE mutations affect the enzymatic activity of NE. However, while most diminish or abrogate NE
proteolytic potential, some mutations appear not to alter the proteolytic function of this protein [22,23] . Given
that uncommon genetic alterations or biochemical features underlie the toxicity of NE mutants, at the
molecular level, the pathogenic effects of NE variants might potentially be attributed to disruption of the NE
protein structure, changes to NE subcellular distribution and/or altered interaction of mutant NE with other
proteins, which overall affect the lifespan of neutrophil precursors, or their maturation or proliferation
ability.
Several, not necessarily mutually exclusive, cellular mechanisms have been proposed to explain the impact
of diverse NE mutations on promoting neutropenia. These include (i) NE misfolding followed by activation
of the unfolded protein response (UPR) and apoptosis of neutrophil precursors; (ii) acute oxidative stress
followed by upregulation of mutant NE levels; (iii) mutation-driven NE mislocalization; and/or (iv) a