Page 632 - Read Online
P. 632
Wilgus. Plast Aesthet Res 2020;7:54 I http://dx.doi.org/10.20517/2347-9264.2020.150 Page 11 of 18
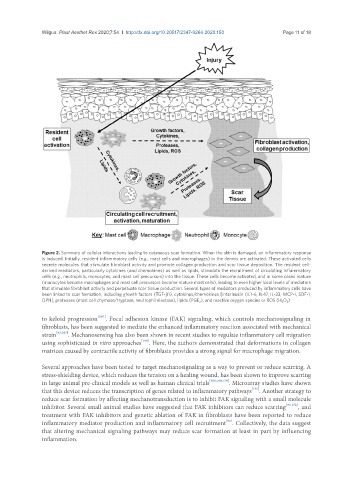
Figure 2. Summary of cellular interactions leading to cutaneous scar formation. When the skin is damaged, an inflammatory response
is induced. Initially, resident inflammatory cells (e.g., mast cells and macrophages) in the dermis are activated. These activated cells
secrete molecules that stimulate fibroblast activity and promote collagen production and scar tissue deposition. The resident cell-
derived mediators, particularly cytokines (and chemokines) as well as lipids, stimulate the recruitment of circulating inflammatory
cells (e.g., neutrophils, monocytes, and mast cell precursors) into the tissue. These cells become activated, and in some cases mature
(monocytes become macrophages and mast cell precursors become mature mast cells), leading to even higher local levels of mediators
that stimulate fibroblast activity and perpetuate scar tissue production. Several types of mediators produced by inflammatory cells have
been linked to scar formation, including growth factors (TGF-β1), cytokines/chemokines [interleukin (IL)-6, IL-17, IL-33, MCP-1, SDF-1,
OPN], proteases (mast cell chymase/tryptase, neutrophil elastase), lipids (PGE 2 ), and reactive oxygen species or ROS (H 2 O 2 )
to keloid progression [167] . Focal adhesion kinase (FAK) signaling, which controls mechanosignaling in
fibroblasts, has been suggested to mediate the enhanced inflammatory reaction associated with mechanical
strain [93,167] . Mechanosensing has also been shown in recent studies to regulate inflammatory cell migration
using sophisticated in vitro approaches [168] . Here, the authors demonstrated that deformations in collagen
matrices caused by contractile activity of fibroblasts provides a strong signal for macrophage migration.
Several approaches have been tested to target mechanosignaling as a way to prevent or reduce scarring. A
stress-shielding device, which reduces the tension on a healing wound, has been shown to improve scarring
in large animal pre-clinical models as well as human clinical trials [166,169,170] . Microarray studies have shown
[171]
that this device reduces the transcription of genes related to inflammatory pathways . Another strategy to
reduce scar formation by affecting mechanotransduction is to inhibit FAK signaling with a small molecule
inhibitor. Several small animal studies have suggested that FAK inhibitors can reduce scarring [93,172] , and
treatment with FAK inhibitors and genetic ablation of FAK in fibroblasts have been reported to reduce
[93]
inflammatory mediator production and inflammatory cell recruitment . Collectively, the data suggest
that altering mechanical signaling pathways may reduce scar formation at least in part by influencing
inflammation.