Page 496 - Read Online
P. 496
Zein et al. Plast Aesthet Res 2020;7:44 I http://dx.doi.org/10.20517/2347-9264.2020.133 Page 5 of 10
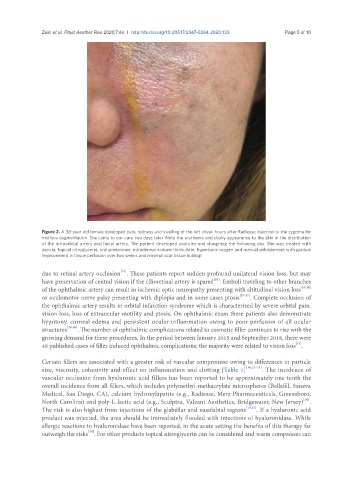
Figure 2. A 38-year old female developed pain, redness and swelling of the left cheek hours after Radiesse injection to the zygoma for
midface augmentation. She came to our care two days later. Note the erythema and dusky appearance to the skin in the distribution
of the infraorbital artery and facial artery. The patient developed pustules and sloughing the following day. She was treated with
aspirin, topical nitroglycerin, oral prednisone, intradermal sodium thiosulfate, hyperbaric oxygen and manual debridement with gradual
improvement in tissue perfusion over two weeks and minimal scar tissue buildup
[21]
due to retinal artery occlusion . These patients report sudden profound unilateral vision loss, but may
[22]
have preservation of central vision if the cilioretinal artery is spared . Emboli traveling to other branches
of the ophthalmic artery can result in ischemic optic neuropathy presenting with altitudinal vision loss [23,24]
or oculomotor nerve palsy presenting with diplopia and in some cases ptosis [25-27] . Complete occlusion of
the ophthalmic artery results in orbital infarction syndrome which is characterized by severe orbital pain,
vision loss, loss of extraocular motility and ptosis. On ophthalmic exam these patients also demonstrate
hypotony, corneal edema and persistent ocular inflammation owing to poor perfusion of all ocular
structures [28-30] . The number of ophthalmic complications related to cosmetic filler continues to rise with the
growing demand for these procedures. In the period between January 2015 and September 2018, there were
[31]
48 published cases of filler induced ophthalmic complications; the majority were related to vision loss .
Certain fillers are associated with a greater risk of vascular compromise owing to differences in particle
size, viscosity, cohesivity and effect on inflammation and clotting [Table 1] [16,32-35] . The incidence of
vascular occlusion from hyaluronic acid fillers has been reported to be approximately one tenth the
overall incidence from all fillers, which includes polymethyl-methacrylate microspheres (Bellafill, Suneva
Medical, San Diego, CA), calcium hydroxylapatite (e.g., Radiesse, Merz Pharmaceuticals, Greensboro,
[36]
North Carolina) and poly-L-lactic acid (e.g., Sculptra, Valeant Aesthetics, Bridgewater, New Jersey) .
The risk is also highest from injections of the glabellar and nasolabial regions [19,37] . If a hyaluronic acid
product was injected, the area should be immediately flooded with injections of hyaluronidase. While
allergic reactions to hyaluronidase have been reported, in the acute setting the benefits of this therapy far
[38]
outweigh the risks . For other products topical nitroglycerin can be considered and warm compresses can