Page 393 - Read Online
P. 393
Paap et al. Plast Aesthet Res 2020;7:36 I http://dx.doi.org/10.20517/2347-9264.2020.121 Page 3 of 11
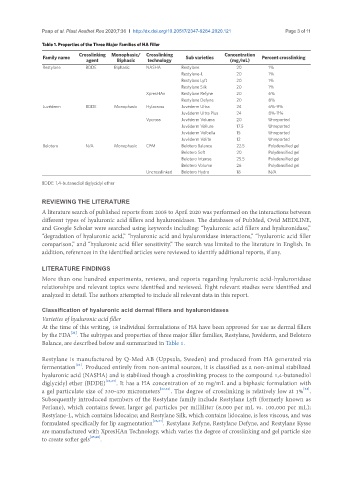
Table 1. Properties of the Three Major Families of HA Filler
Crosslinking Monophasic/ Crosslinking Concentration
Family name agent Biphasic technology Sub varieties (mg/mL) Percent crosslinking
Restylane BDDE Biphasic NASHA Restylane 20 1%
Restylane-L 20 1%
Restylane Lyft 20 1%
Restylane Silk 20 1%
XpresHAn Restylane Refyne 20 6%
Restylane Defyne 20 8%
Juvéderm BDDE Monophasic Hylacross Juvéderm Ultra 24 6%-9%
Juvéderm Ultra Plus 24 8%-11%
Vycross Juvéderm Voluma 20 Unreported
Juvéderm Vollure 17.5 Unreported
Juvéderm Volbella 15 Unreported
Juvéderm Volite 12 Unreported
Belotero N/A Monophasic CPM Belotero Balance 22.5 Polydensified gel
Belotero Soft 20 Polydensified gel
Belotero Intense 25.5 Polydensified gel
Belotero Volume 26 Polydensified gel
Uncrosslinked Belotero Hydro 18 N/A
BDDE: 1,4-butanediol diglycidyl ether
REVIEWING THE LITERATURE
A literature search of published reports from 2005 to April 2020 was performed on the interactions between
different types of hyaluronic acid fillers and hyaluronidases. The databases of PubMed, Ovid MEDLINE,
and Google Scholar were searched using keywords including: “hyaluronic acid fillers and hyaluronidase,”
“degradation of hyaluronic acid,” “hyaluronic acid and hyaluronidase interactions,” “hyaluronic acid filler
comparison,” and “hyaluronic acid filler sensitivity.” The search was limited to the literature in English. In
addition, references in the identified articles were reviewed to identify additional reports, if any.
LITERATURE FINDINGS
More than one hundred experiments, reviews, and reports regarding hyaluronic acid-hyaluronidase
relationships and relevant topics were identified and reviewed. Eight relevant studies were identified and
analyzed in detail. The authors attempted to include all relevant data in this report.
Classification of hyaluronic acid dermal fillers and hyaluronidases
Varieties of hyaluronic acid filler
At the time of this writing, 18 individual formulations of HA have been approved for use as dermal fillers
by the FDA . The subtypes and properties of three major filler families, Restylane, Juvéderm, and Belotero
[21]
Balance, are described below and summarized in Table 1.
Restylane is manufactured by Q-Med AB (Uppsala, Sweden) and produced from HA generated via
fermentation . Produced entirely from non-animal sources, it is classified as a non-animal stabilized
[22]
hyaluronic acid (NASHA) and is stabilized though a crosslinking process to the compound 1,4-butanediol
diglycidyl ether (BDDE) [22,23] . It has a HA concentration of 20 mg/mL and a biphasic formulation with
[24]
a gel particulate size of 330-430 micrometers [22,24] . The degree of crosslinking is relatively low at 1% .
Subsequently introduced members of the Restylane family include Restylane Lyft (formerly known as
Perlane), which contains fewer, larger gel particles per milliliter (8,000 per mL vs. 100,000 per mL);
Restylane-L, which contains lidocaine; and Restylane Silk, which contains lidocaine, is less viscous, and was
formulated specifically for lip augmentation [22,24] . Restylane Refyne, Restylane Defyne, and Restylane Kysse
are manufactured with XpresHAn Technology, which varies the degree of crosslinking and gel particle size
to create softer gels [25,26] .