Page 326 - Read Online
P. 326
Moores et al. Plast Aesthet Res 2018;5:44 I http://dx.doi.org/10.20517/2347-9264.2018.32 Page 3 of 6
Figure 1. A midline laparotomy is seen, in addition to a serially debrided groin wound which contains the bulk of the cryovein bypass graft
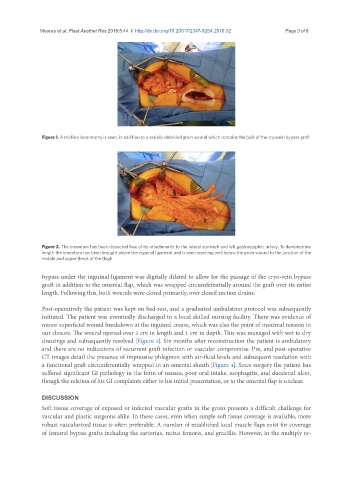
Figure 2. The omentum has been dissected free of its attachments to the lateral stomach and left gastroepiploic artery. To demonstrate
length the omentum has been brought above the inguinal ligament and is seen reaching well below the groin wound to the junction of the
middle and upper thirds of the thigh
bypass under the inguinal ligament was digitally dilated to allow for the passage of the cryo-vein bypass
graft in addition to the omental flap, which was wrapped circumferentially around the graft over its entire
length. Following this, both wounds were closed primarily, over closed suction drains.
Post-operatively the patient was kept on bed-rest, and a graduated ambulation protocol was subsequently
initiated. The patient was eventually discharged to a local skilled nursing facility. There was evidence of
minor superficial wound breakdown at the inguinal crease, which was also the point of maximal tension in
our closure. The wound opened over 2 cm in length and 1 cm in depth. This was managed with wet to dry
dressings and subsequently resolved [Figure 3]. Six months after reconstruction the patient is ambulatory
and there are no indications of recurrent graft infection or vascular compromise. Pre, and post-operative
CT images detail the presence of impressive phlegmon with air-fluid levels and subsequent resolution with
a functional graft circumferentially wrapped in an omental sheath [Figure 4]. Since surgery the patient has
suffered significant GI pathology in the form of nausea, poor oral intake, esophagitis, and duodenal ulcer,
though the relation of his GI complaints either to his initial presentation, or to the omental flap is unclear.
DISCUSSION
Soft tissue coverage of exposed or infected vascular grafts in the groin presents a difficult challenge for
vascular and plastic surgeons alike. In these cases, even when simple soft tissue coverage is available, more
robust vascularized tissue is often preferable. A number of established local muscle flaps exist for coverage
of femoral bypass grafts including the sartorius, rectus femoris, and gracillis. However, in the multiply re-