Page 128 - Read Online
P. 128
Plastic and Aesthetic Research
A unique late complication with the use
of calcium hydroxylapatite fi ller in facial
lipoatrophy rehabilitation
Raffaele Rauso 1,2
1 Univeristy of Foggia, 71121 Foggia, Italy.
2 Department of Plastic Surgery, La Sapienza University, 00185 Rome, Italy.
Address for correspondence: Dr. Raffaele Rauso, Centro Polispecialistico Santa Apollonia, Department of Aesthetic Plastic and Craniofacial
Surgery, Via Martiri del Dissenso 47, 81055 Santa Maria Capua Vetere CE, Italy. E-mail: dr.raffaele.rauso@gmail.com
Sir,
Radiesse (Merz Aesthetics, Franksville, WI, USA) is an
injectable filler material composed of synthetic calcium
hydroxylapatite (CaHA) microspheres suspended in an
aqueous carrier gel. Cosmetic use of Radiesse in facial
rejuvenation is well-known. Treatment sites amenable
to calcium hydroxylapatite (CH) injection include the
naso-labial folds, marionette lines, perioral lines,
prejowl sulcus, zygoma and malar eminence, tear trough
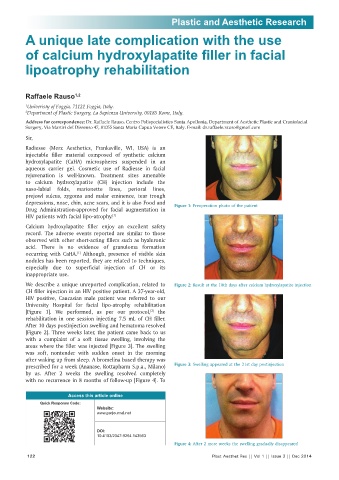
depressions, nose, chin, acne scars, and it is also Food and Figure 1: Preoperation photo of the patient
Drug Administration-approved for facial augmentation in
HIV patients with facial lipo-atrophy. [1]
Calcium hydroxylapatite filler enjoy an excellent safety
record. The adverse events reported are similar to those
observed with other short-acting fillers such as hyaluronic
acid. There is no evidence of granuloma formation
occurring with CaHA. Although, presence of visible skin
[1]
nodules has been reported, they are related to techniques,
especially due to superficial injection of CH or its
inappropriate use.
We describe a unique unreported complication, related to Figure 2: Result at the 10th days after calcium hydroxylapatite injection
CH filler injection in an HIV positive patient. A 37-year-old,
HIV positive, Caucasian male patient was referred to our
University Hospital for facial lipo-atrophy rehabilitation
[2]
[Figure 1]. We performed, as per our protocol, the
rehabilitation in one session injecting 7.5 mL of CH filler.
After 10 days postinjection swelling and hematoma resolved
[Figure 2]. Three weeks later, the patient came back to us
with a complaint of a soft tissue swelling, involving the
areas where the filler was injected [Figure 3]. The swelling
was soft, nontender with sudden onset in the morning
after waking up from sleep. A bromelina based therapy was
prescribed for a week (Ananase, Rottapharm S.p.a., Milano) Figure 3: Swelling appeared at the 21st day postinjection
by us. After 2 weeks the swelling resolved completely
with no recurrence in 8 months of follow-up [Figure 4]. To
Access this article online
Quick Response Code:
Website:
www.parjournal.net
DOI:
10.4103/2347-9264.143563
Figure 4: After 2 more weeks the swelling gradually disappeared
122 Plast Aesthet Res || Vol 1 || Issue 3 || Dec 2014