Page 34 - Read Online
P. 34
Philips et al. Plast Aesthet Res 2022;9:4 https://dx.doi.org/10.20517/2347-9264.2021.83 Page 3 of 10
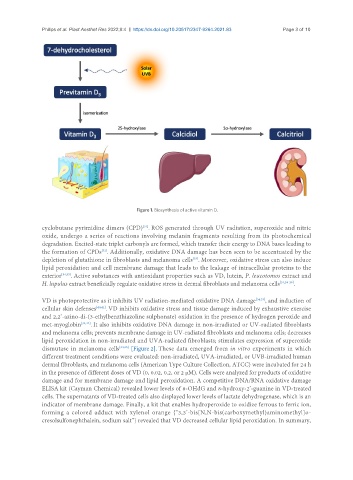
Figure 1. Biosynthesis of active vitamin D.
[31]
cyclobutane pyrimidine dimers (CPD) . ROS generated through UV radiation, superoxide and nitric
oxide, undergo a series of reactions involving melanin fragments resulting from its photochemical
degradation. Excited-state triplet carbonyls are formed, which transfer their energy to DNA bases leading to
the formation of CPDs . Additionally, oxidative DNA damage has been seen to be accentuated by the
[32]
[33]
depletion of glutathione in fibroblasts and melanoma cells . Moreover, oxidative stress can also induce
lipid peroxidation and cell membrane damage that leads to the leakage of intracellular proteins to the
exterior [34,35] . Active substances with antioxidant properties such as VD, lutein, P. leucotomos extract and
H. lupulus extract beneficially regulate oxidative stress in dermal fibroblasts and melanoma cells [31,34-39] .
VD is photoprotective as it inhibits UV radiation-mediated oxidative DNA damage [34,35] , and induction of
cellular skin defenses [40,41] . VD inhibits oxidative stress and tissue damage induced by exhaustive exercise
and 2,2’-azino-di-(3-ethylbenzthiazoline sulphonate) oxidation in the presence of hydrogen peroxide and
met-myoglobin [34,42] . It also inhibits oxidative DNA damage in non-irradiated or UV-radiated fibroblasts
and melanoma cells; prevents membrane damage in UV-radiated fibroblasts and melanoma cells; decreases
lipid peroxidation in non-irradiated and UVA-radiated fibroblasts; stimulates expression of superoxide
dismutase in melanoma cells [34,35] [Figure 2]. These data emerged from in vitro experiments in which
different treatment conditions were evaluated: non-irradiated, UVA-irradiated, or UVB-irradiated human
dermal fibroblasts, and melanoma cells (American Type Culture Collection, ATCC) were incubated for 24 h
in the presence of different doses of VD (0, 0.02, 0.2, or 2 μM). Cells were analyzed for products of oxidative
damage and for membrane damage and lipid peroxidation. A competitive DNA/RNA oxidative damage
ELISA kit (Cayman Chemical) revealed lower levels of 8-OHdG and 8-hydroxy-2’-guanine in VD-treated
cells. The supernatants of VD-treated cells also displayed lower levels of lactate dehydrogenase, which is an
indicator of membrane damage. Finally, a kit that enables hydroperoxide to oxidize ferrous to ferric ion,
forming a colored adduct with xylenol orange {“3,3’-bis[N,N-bis(carboxymethyl)aminomethyl]o-
cresolsulfonephthalein, sodium salt”} revealed that VD decreased cellular lipid peroxidation. In summary,