Page 36 - Read Online
P. 36
Page 2 of 10 Polykandriotis et al. Plast Aesthet Res 2018;5:37 I http://dx.doi.org/10.20517/2347-9264.2018.52
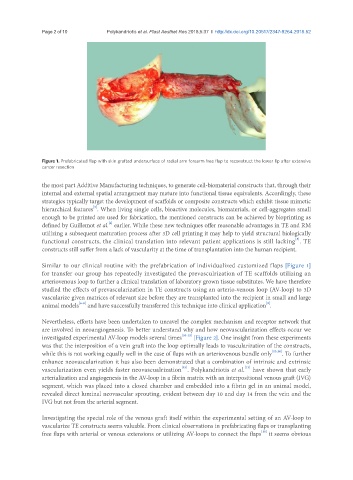
Figure 1. Prefabricated flap with skin grafted undersurface of radial arm forearm free flap to reconstruct the lower lip after extensive
cancer resection
the most part Additive Manufacturing techniques, to generate cell-biomaterial constructs that, through their
internal and external spatial arrangement may mature into functional tissue equivalents. Accordingly, these
strategies typically target the development of scaffolds or composite constructs which exhibit tissue mimetic
[3]
hierarchical features . When living single cells, bioactive molecules, biomaterials, or cell-aggregates small
enough to be printed are used for fabrication, the mentioned constructs can be achieved by bioprinting as
[4]
defined by Guillemot et al. earlier. While these new techniques offer reasonable advantages in TE and RM
utilizing a subsequent maturation process after 3D cell printing it may help to yield structural biologically
[5]
functional constructs, the clinical translation into relevant patient applications is still lacking . TE
constructs still suffer from a lack of vascularity at the time of transplantation into the human recipient.
Similar to our clinical routine with the prefabrication of individualized customized flaps [Figure 1]
for transfer our group has repeatedly investigated the prevasculrization of TE scaffolds utilizing an
arteriovenous loop to further a clinical translation of laboratory grown tissue substitutes. We have therefore
studied the effects of prevascularization in TE constructs using an arterio-venous loop (AV-loop) to 3D
vascularize given matrices of relevant size before they are transplanted into the recipient in small and large
[9]
[6-8]
animal models and have successfully transferred this technique into clinical application .
Nevertheless, efforts have been undertaken to unravel the complex mechanism and receptor network that
are involved in neoangiogenesis. To better understand why and how neovascularization effects occur we
investigated experimental AV-loop models several times [10-12] [Figure 2]. One insight from these experiments
was that the interposition of a vein graft into the loop optimally leads to vascularization of the constructs,
while this is not working equally well in the case of flaps with an arteriovenous bundle only [13,14] . To further
enhance neovascularization it has also been demonstrated that a combination of intrinsic and extrinsic
[13]
[15]
vascularization even yields faster neovascualrization . Polykandriotis et al. have shown that early
arterialization and angiogenesis in the AV-loop in a fibrin matrix with an interpositional venous graft (IVG)
segment, which was placed into a closed chamber and embedded into a fibrin gel in an animal model,
revealed direct luminal neovascular sprouting, evident between day 10 and day 14 from the vein and the
IVG but not from the arterial segment.
Investigating the special role of the venous graft itself within the experimental setting of an AV-loop to
vascularize TE constructs seems valuable. From clinical observations in prefabricating flaps or transplanting
[16]
free flaps with arterial or venous extensions or utilizing AV-loops to connect the flaps it seems obvious