Page 60 - Read Online
P. 60
Page 10 of 13 Rajaram et al. Plast Aesthet Res. 2025;12:6 https://dx.doi.org/10.20517/2347-9264.2024.147
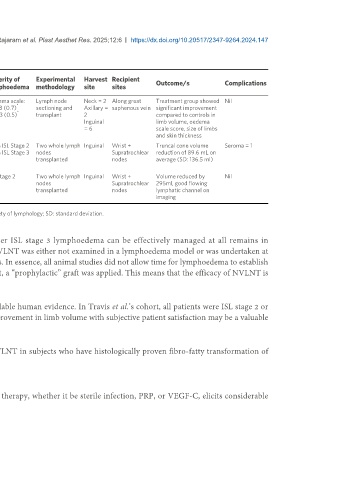
Table 2. Study characteristics of included human trials of lymph node grafting
Number
Study Site of Time since Severity of Experimental Harvest Recipient
Author Year of Aetiology Outcome/s Complications
design disease resection lymphoedema methodology site sites
patients
[20]
Belcaro et al. 2008 Prospective 17 Lower Non-cancer, > 5 years Oedema scale: Lymph node Neck = 2 Along great Treatment group showed Nil
*
limb nil radiation T: 4.3 (0.7) sectioning and Axillary = saphenous vein significant improvement
*
C: 4.3 (0.5) transplant 2 compared to controls in
Inguinal limb volume, oedema
= 6 scale score, size of limbs
and skin thickness
[21]
Travis et al. 2015 Prospective 10 Upper Axillary > 6 months 60% ISL Stage 2 Two whole lymph Inguinal Wrist + Truncal cone volume Seroma = 1
limb dissection (mentioning 40% ISL Stage 3 nodes Supratrochlear reduction of 89.6 mL on
stable lympho- transplanted nodes average (SD: 136.5 ml)
edema)
[22]
Brian and McEwan 2017 Case report 1 Upper Axillary 1 year ISL Stage 2 Two whole lymph Inguinal Wrist + Volume reduced by Nil
limb dissection nodes Supratrochlear 295ml, good flowing
transplanted nodes lymphatic channel on
imaging
*
Data presented as mean (standard deviation). T: Treatment group; C: control group; ISL: international society of lymphology; SD: standard deviation.
and the resultant swelling is especially difficult to manage. In fact, whether ISL stage 3 lymphoedema can be effectively managed at all remains in
question [23,24,25] . Within all animal models that have currently been explored, NVLNT was either not examined in a lymphoedema model or was undertaken at
the time of surgical disruption of lymphatic networks in the control populations. In essence, all animal studies did not allow time for lymphoedema to establish
within animal models before examining the efficacy of NVLNT, and as a result, a “prophylactic” graft was applied. This means that the efficacy of NVLNT is
not readily validated in established lymphoedema even within animal models.
This is especially pertinent when considering the study population of the available human evidence. In Travis et al.’s cohort, all patients were ISL stage 2 or
higher, with 40% of patients being ISL stage 3 . Given this, even a modest improvement in limb volume with subjective patient satisfaction may be a valuable
[21]
outcome in the most stubborn and difficult-to-treat form of lymphoedema.
Therefore, future animal models could attempt to measure the efficacy of NVLNT in subjects who have histologically proven fibro-fatty transformation of
lymphoedema.
No adjuvants used
Secondly, most animal studies demonstrate that the addition of any adjuvant therapy, whether it be sterile infection, PRP, or VEGF-C, elicits considerable