Page 14 - Read Online
P. 14
Yesantharao et al. Plast Aesthet Res 2022;9:60 https://dx.doi.org/10.20517/2347-9264.2022.67 Page 7 of 13
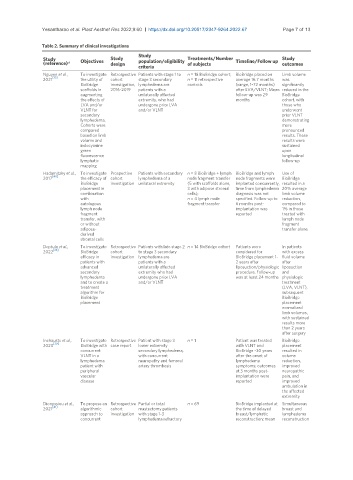
Table 2. Summary of clinical investigations
Study
Study Objectives Study population/eligibility Treatments/Number Timeline/Follow up Study
(reference)* design criteria of subjects outcomes
Nguyen et al., To investigate Retrospective Patients with stage 1 to n = 18 BioBridge cohort; BioBridge placed on Limb volume
[35]
2021 the utility of cohort stage 3 secondary n = 11 retrospective average 16.7 months was
BioBridge investigation, lymphedema are controls (range, 1-72 months) significantly
scaffolds in 2016-2019 patients with a after LVA/VLNT; Mean reduced in the
augmenting unilaterally affected follow-up was 29 BioBridge
the effects of extremity, who had months cohort, with
LVA and/or undergone prior LVA those who
VLNT for and/or VLNT underwent
secondary prior VLNT
lymphedema. demonstrating
Cohorts were more
compared pronounced
based on limb results. These
volume and results were
indocyanine sustained
green upon
fluorescence longitudinal
lymphatic follow-up
mapping
Hadamitzky et al., To investigate Prospective Patients with secondary n = 8 BioBridge + lymph BioBridge and lymph Use of
2017 [40] the efficacy of cohort lymphedema of a node fragment transfer node fragments were BioBridge
BioBridge investigation unilateral extremity (5 with scaffolds alone, implanted concurrently, resulted in a
placement in 3 with adipose stromal time from lymphedema 20% average
combination cells); diagnosis was not limb volume
with n = 4 lymph node specified. Follow-up to reduction,
autologous fragment transfer 6 months post- compared to
lymph node implantation was 1% in those
fragment reported treated with
transfer, with lymph node
or without fragment
adipose- transfer alone
derived
stromal cells
Deptula et al., To investigate Retrospective Patients with late stage 2 n = 14 BioBridge cohort Patients were In patients
[36]
2022 BioBridge cohort to stage 3 secondary considered for with excess
efficacy in investigation lymphedema are BioBridge placement 1- fluid volume
patients with patients with a 2 years after after
advanced unilaterally affected liposuction/physiologic liposuction
secondary extremity who had procedure. Follow-up and
lymphedema undergone prior LVA was at least 24 months physiologic
and to create a and/or VLNT treatment
treatment (LVA, VLNT),
algorithm for subsequent
BioBridge BioBridge
placement placement
normalized
limb volumes,
with sustained
results more
than 2 years
after surgery
Inchauste et al., To investigate Retrospective Patient with stage 3 n = 1 Patient was treated BioBridge
2020 [39] BioBridge with case report lower extremity with VLNT and placement
concurrent secondary lymphedema, BioBridge ~30 years resulted in
VLNT in a with concurrent after the onset of volume
lymphedema neuropathy and femoral lymphedema reduction,
patient with artery thrombosis symptoms; outcomes improved
peripheral at 3 months post- neuropathic
vascular implantation were pain, and
disease reported improved
ambulation in
the affected
extremity
Dionyssiou et al., To propose an Retrospective Partial or total n = 69 BioBridge implanted at Simultaneous
[41]
2021 algorithmic cohort mastectomy patients the time of delayed breast and
approach to investigation with stage 1-3 breast/lymphatic lymphedema
concurrent lymphedema refractory reconstruction; mean reconstruction