Page 82 - Read Online
P. 82
Slattery et al. Neuroimmunol Neuroinflammation 2018;5:11 I http://dx.doi.org/10.20517/2347-8659.2018.05 Page 3 of 15
Liver CNS
a
Cardiovascular system
a
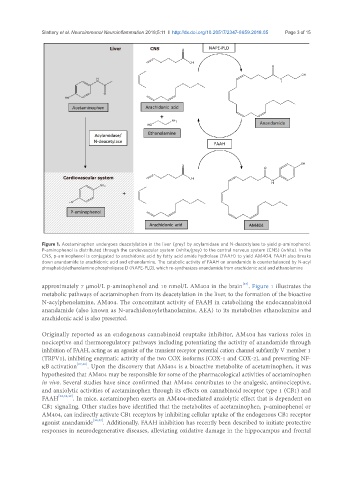
Figure 1. Acetaminophen undergoes deacetylation in the liver (grey) by acylamidase and N-deacetylase to yield p-aminophenol.
P-aminophenol is distributed through the cardiovascular system (white/grey) to the central nervous system (CNS) (white). In the
CNS, p-aminophenol is conjugated to arachidonic acid by fatty acid amide hydrolase (FAAH) to yield AM404. FAAH also breaks
down anandamide to arachidonic acid and ethanolamine. The catabolic activity of FAAH on anandamide is counterbalanced by N-acyl
phosphatidylethanolamine phospholipase D (NAPE-PLD), which re-synthesizes anandamide from arachidonic acid and ethanolamine
[27]
approximately 7 µmol/L p-aminophenol and 10 nmol/L AM404 in the brain . Figure 1 illustrates the
metabolic pathways of acetaminophen from its deacetylation in the liver, to the formation of the bioactive
N-acylphenolamine, AM404. The concomitant activity of FAAH in catabolizing the endocannabinoid
anandamide (also known as N-arachidonoylethanolamine, AEA) to its metabolites ethanolamine and
arachidonic acid is also presented.
Originally reported as an endogenous cannabinoid reuptake inhibitor, AM404 has various roles in
nociceptive and thermoregulatory pathways including potentiating the activity of anandamide through
inhibition of FAAH, acting as an agonist of the transient receptor potential cation channel subfamily V member 1
(TRPV1), inhibiting enzymatic activity of the two COX isoforms (COX-1 and COX-2), and preventing NF-
κB activation [27,30] . Upon the discovery that AM404 is a bioactive metabolite of acetaminophen, it was
hypothesized that AM404 may be responsible for some of the pharmacological activities of acetaminophen
in vivo. Several studies have since confirmed that AM404 contributes to the analgesic, antinociceptive,
and anxiolytic activities of acetaminophen through its effects on cannabinoid receptor type 1 (CB1) and
FAAH [22,31,32] . In mice, acetaminophen exerts an AM404-mediated anxiolytic effect that is dependent on
CB1 signaling. Other studies have identified that the metabolites of acetaminophen, p-aminophenol or
AM404, can indirectly activate CB1 receptors by inhibiting cellular uptake of the endogenous CB1 receptor
agonist anandamide [32,33] . Additionally, FAAH inhibition has recently been described to initiate protective
responses in neurodegenerative diseases, alleviating oxidative damage in the hippocampus and frontal