Page 270 - Read Online
P. 270
Page 2 of 13 Dello Russo et al. Neuroimmunol Neuroinflammation 2018;5:36 I http://dx.doi.org/10.20517/2347-8659.2018.42
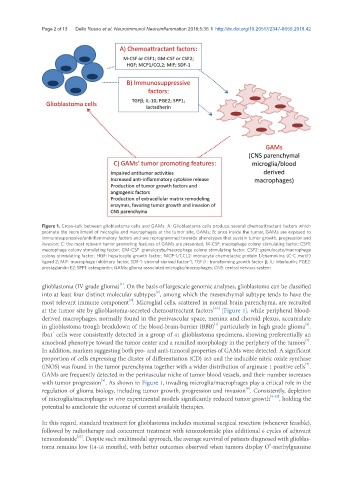
Figure 1. Cross-talk between glioblastoma cells and GAMs. A: Glioblastoma cells produce several chemoattractant factors which
promote the recruitment of microglia and macrophages at the tumor site, GAMs; B: once inside the tumor, GAMs are exposed to
immunosuppressive/antinflammatory factors and are reprogrammed towards phenotypes that sustain tumor growth, progression and
invasion; C: the most relevant tumor promoting features of GAMs are presented. M-CSF: macrophage colony stimulating factor; CSF1:
macrophage colony stimulating factor; GM-CSF: granulocyte/macrophage colony stimulating factor; CSF2: granulocyte/macrophage
colony stimulating factor; HGF: hepatocyte growth factor; MCP-1/CCL2: monocyte chemotactic protein 1/chemokine (C-C motif)
ligand 2; MIF: macrophage inhibitory factor; SDF-1: stromal-derived factor-1; TGFβ: transforming growth factor β; IL: interleukin; PGE2:
prostaglandin E2; SPP1: osteopontin; GAMs: glioma associated microglia/macrophages; CNS: central nervous system
[1]
glioblastoma (IV grade glioma) . On the basis of largescale genomic analyses, glioblastoma can be classified
[2]
into at least four distinct molecular subtypes , among which the mesenchymal subtype tends to have the
[3]
most relevant immune component . Microglial cells, scattered in normal brain parenchyma, are recruited
[4,5]
at the tumor site by glioblastoma-secreted chemoattractant factors [Figure 1], while peripheral blood-
derived macrophages, normally found in the perivascular space, meninx and choroid plexus, accumulate
[6]
[1]
in glioblastoma trough breakdown of the blood-brain-barrier (BBB) particularly in high grade glioma .
Iba1 cells were consistently detected in a group of 41 glioblastoma specimens, showing preferentially an
+
[7]
amoeboid phenotype toward the tumor center and a ramified morphology in the periphery of the tumors .
In addition, markers suggesting both pro- and anti-tumoral properties of GAMs were detected. A significant
proportion of cells expressing the cluster of differentiation (CD) 163 and the inducible nitric oxide synthase
[7]
(iNOS) was found in the tumor parenchyma together with a wider distribution of arginase 1 positive cells .
GAMs are frequently detected in the perivascular niche of tumor blood vessels, and their number increases
[8]
with tumor progression . As shown in Figure 1, invading microglia/macrophages play a critical role in the
[8]
regulation of glioma biology, including tumor growth, progression and invasion . Consistently, depletion
of microglia/macrophages in vivo experimental models significantly reduced tumor growth [8-12] , holding the
potential to ameliorate the outcome of current available therapies.
In this regard, standard treatment for glioblastoma includes maximal surgical resection (whenever feasible),
followed by radiotherapy and concurrent treatment with temozolomide plus additional 6 cycles of adjuvant
[13]
temozolomide . Despite such multimodal approach, the average survival of patients diagnosed with glioblas-
toma remains low (14-16 months), with better outcomes observed when tumors display O -methylguanine
6