Page 24 - Read Online
P. 24
Page 6 of 11 Mathieu et al. Metab Target Organ Damage 2022;2:15 https://dx.doi.org/10.20517/mtod.2022.16
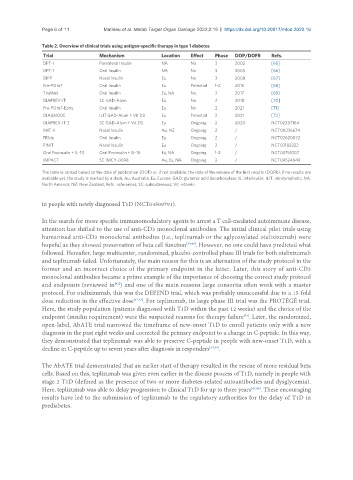
Table 2. Overview of clinical trials using antigen-specific therapy in type 1 diabetes
Trial Mechanism Location Effect Phase DOP/DOFR Refs.
DPT-1 Parenteral Insulin NA No 3 2002 [65]
DPT-1 Oral Insulin NA No 3 2005 [66]
DIPP Nasal Insulin Eu No 3 2008 [67]
Pre-POInT Oral Insulin Eu Potential 1-2 2015 [68]
TrialNet Oral Insulin Eu, NA No 3 2017 [69]
DIAPREV-IT SC GAD-Alum Eu No 2 2018 [70]
Pre-POInT-Early Oral Insulin Eu No 2 2021 [71]
DIAGNODE ILIT GAD-Alum + Vit D3 Eu Potential 2 2021 [72]
DIAPREV-IT 2 SC GAD-Alum + Vit D3 Eu Ongoing 2 2020 NCT02387164
INIT-II Nasal Insulin Au, NZ Ongoing 2 / NCT00336674
FR1da Oral Insulin Eu Ongoing 2 / NCT02620072
PINIT Nasal Insulin Eu Ongoing 2 / NCT03182322
Oral Proinsulin + IL-10 Oral Proinsulin + IL-10 Eu, NA Ongoing 1-2 / NCT03751007
IMPACT SC IMCY-0098 Au, Eu, NA Ongoing 2 / NCT04524949
The table is ranked based on the date of publication (DOP) or, if not available, the date of the release of the first results (DOFR). If no results are
available yet, the study is marked by a dash. Au: Australia; Eu: Europe; GAD: glutamic acid decarboxylase; IL: interleukin; ILIT: intralymphatic; NA:
North America; NZ: New Zealand; Refs.: references; SC: subcutaneous; Vit: vitamin.
in people with newly diagnosed T1D (NCT04509791).
In the search for more specific immunomodulatory agents to arrest a T cell-mediated autoimmune disease,
attention has shifted to the use of anti-CD3 monoclonal antibodies. The initial clinical pilot trials using
humanized anti-CD3 monoclonal antibodies (i.e., teplizumab or the aglycosylated otelixizumab) were
hopeful as they showed preservation of beta cell function [39,40] . However, no one could have predicted what
followed. Hereafter, large multicenter, randomized, placebo-controlled phase III trials for both otelixizumab
and teplizumab failed. Unfortunately, the main reason for this is an alternation of the study protocol in the
former and an incorrect choice of the primary endpoint in the latter. Later, this story of anti-CD3
monoclonal antibodies became a prime example of the importance of choosing the correct study protocol
[41]
and endpoints (reviewed in ) and one of the main reasons large consortia often work with a master
protocol. For otelixizumab, this was the DEFEND trial, which was probably unsuccessful due to a 15-fold
dose reduction in the effective dose [42,43] . For teplizumab, its large phase III trial was the PROTÉGÉ trial.
Here, the study population (patients diagnosed with T1D within the past 12 weeks) and the choice of the
endpoint (insulin requirement) were the suspected reasons for therapy failure . Later, the randomized,
[44]
open-label, AbATE trial narrowed the timeframe of new-onset T1D to enroll patients only with a new
diagnosis in the past eight weeks and corrected the primary endpoint to a change in C-peptide. In this way,
they demonstrated that teplizumab was able to preserve C-peptide in people with new-onset T1D, with a
decline in C-peptide up to seven years after diagnosis in responders [45,46] .
The AbATE trial demonstrated that an earlier start of therapy resulted in the rescue of more residual beta
cells. Based on this, teplizumab was given even earlier in the disease process of T1D, namely in people with
stage 2 T1D (defined as the presence of two or more diabetes-related autoantibodies and dysglycemia).
Here, teplizumab was able to delay progression to clinical T1D for up to three years [47,48] . These encouraging
results have led to the submission of teplizumab to the regulatory authorities for the delay of T1D in
prediabetes.