Page 104 - Read Online
P. 104
Page 6 of 13 Mauri et al. Mini-invasive Surg 2022;6:49 https://dx.doi.org/10.20517/2574-1225.2022.34
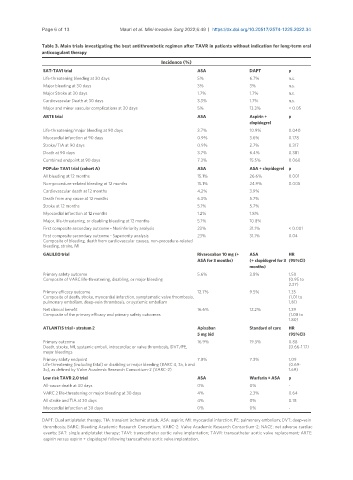
Table 3. Main trials investigating the best antithrombotic regimen after TAVR in patients without indication for long-term oral
anticoagulant therapy
Incidence (%)
SAT-TAVI trial ASA DAPT p
Life-threatening bleeding at 30 days 5% 6.7% n.s.
Major bleeding at 30 days 3% 3% n.s.
Major Stroke at 30 days 1.7% 1.7% n.s.
Cardiovascular Death at 30 days 3.3% 1.7% n.s.
Major and minor vascular complications at 30 days 5% 13.3% < 0.05
ARTE trial ASA Aspirin + p
clopidogrel
Life-threatening/major bleeding at 90 days 3.7% 10.9% 0.040
Myocardial infarction at 90 days 0.9% 3.6% 0.178
Stroke/TIA at 90 days 0.9% 2.7% 0.317
Death at 90 days 3.7% 6.4% 0.381
Combined endpoint at 90 days 7.3% 15.5% 0.060
POPular TAVI trial (cohort A) ASA ASA + clopidogrel p
All bleeding at 12 months 15.1% 26.6% 0.001
Non-procedure-related bleeding at 12 months 15.1% 24.9% 0.005
Cardiovascular death at 12 months 4.2% 3.9%
Death from any cause at 12 months 6.3% 5.7%
Stroke at 12 months 5.1% 5.7%
Myocardial infarction at 12 months 1.2% 1.8%
Major, life-threatening, or disabling bleeding at 12 months 5.1% 10.8%
First composite secondary outcome - Noninferiority analysis 23% 31.1% < 0.001
First composite secondary outcome - Superiority analysis 23% 31.1% 0.04
Composite of bleeding, death from cardiovascular causes, non-procedure-related
bleeding, stroke, MI
GALILEO trial Rivaroxaban 10 mg (+ ASA HR
ASA for 3 months) (+ clopidogrel for 3 (95%CI)
months)
Primary safety outcome 5.6% 3.8% 1.50
Composite of VARC life-threatening, disabling, or major bleeding (0.95 to
2.37)
Primary efficacy outcome 12.7% 9.5% 1.35
Composite of death, stroke, myocardial infarction, symptomatic valve thrombosis, (1.01 to
pulmonary embolism, deep-vein thrombosis, or systemic embolism 1.81)
Net clinical benefit 16.6% 12.2% 1.39
Composite of the primary efficacy and primary safety outcomes (1.08 to
1.80)
ATLANTIS trial - stratum 2 Apixaban Standard of care HR
5 mg bid (95%CI)
Primary outcome 16.9% 19.3% 0.88
Death, stroke, MI, systemic emboli, intracardiac or valve thrombosis, DVT/PE, (0.66-1.17)
major bleedings
Primary safety endpoint 7.8% 7.3% 1.09
Life-threatening (including fatal) or disabling or major bleeding (BARC 4, 3a, b and (0.69-
3c), as defined by Valve Academic Research Consortium-2 (VARC-2) 1.69)
Low risk TAVR 2.0 trial ASA Warfarin + ASA p
All-cause death at 30 days 0% 0% -
VARC 2 life-threatening or major bleeding at 30 days 4% 2.3% 0.64
All stroke and TIA at 30 days 4% 0% 0.18
Myocardial infarction at 30 days 0% 0% -
DAPT: Dual antiplatelet therapy; TIA: transient ischemic attack; ASA: aspirin; MI: myocardial infarction; PE: pulmonary embolism; DVT: deep-vein
thrombosis; BARC: Bleeding Academic Research Consortium; VARC-2: Valve Academic Research Consortium-2; NACE: net adverse cardiac
events; SAT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation; TAVR: transcatheter aortic valve replacement; ARTE:
aspirin versus aspirin + clopidogrel following transcatheter aortic valve implantation.