Page 111 - Read Online
P. 111
Hasson et al. Mini-invasive Surg 2020;4:46 I http://dx.doi.org/10.20517/2574-1225.2020.10 Page 5 of 16
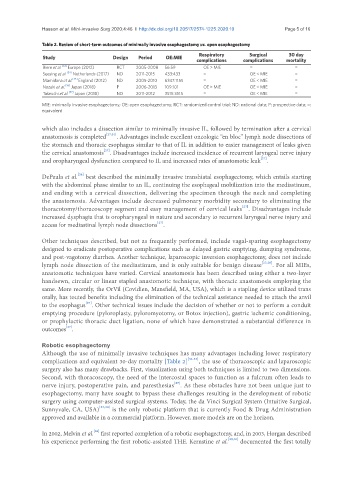
Table 2. Review of short-term outcomes of minimally invasive esophagectomy vs. open esophagectomy
Respiratory Surgical 30 day
Study Design Period OE:MIE complications complications mortality
Biere et al. [33] Europe (2012) RCT 2005-2008 56:59 OE > MIE = =
Seesing et al. [34] Netherlands (2017) ND 2011-2015 433:433 = OE < MIE =
Mamidana et al. [35] England (2012) ND 2005-2010 6347:1155 = OE < MIE =
Nozaki et al. [36] Japan (2018) P 2006-2013 109:101 OE > MIE OE < MIE =
Takeuchi et al. [37] Japan (2018) ND 2011-2012 3515:3515 = OE < MIE =
MIE: minimally invasive esophagectomy; OE: open esophagectomy; RCT: randomized control trial; ND: national data; P: prospective data; =:
equivalent
which also includes a dissection similar to minimally invasive IL, followed by termination after a cervical
anastomosis is completed [27,31] . Advantages include excellent oncologic “en bloc” lymph node dissections of
the stomach and thoracic esophagus similar to that of IL in addition to easier management of leaks given
[27]
the cervical anastomosis . Disadvantages include increased incidence of recurrent laryngeal nerve injury
[27]
and oropharyngeal dysfunction compared to IL and increased rates of anastomotic leak .
[26]
DePaula et al. best described the minimally invasive transhiatal esophagectomy, which entails starting
with the abdominal phase similar to an IL, continuing the esophageal mobilization into the mediastinum,
and ending with a cervical dissection, delivering the specimen through the neck and completing
the anastomosis. Advantages include decreased pulmonary morbidity secondary to eliminating the
[27]
thoracotomy/thoracoscopy segment and easy management of cervical leaks . Disadvantages include
increased dysphagia that is oropharyngeal in nature and secondary to recurrent laryngeal nerve injury and
[27]
access for mediastinal lymph node dissections .
Other techniques described, but not as frequently performed, include vagal-sparing esophagectomy
designed to eradicate postoperative complications such as delayed gastric emptying, dumping syndrome,
and post-vagotomy diarrhea. Another technique, laparoscopic inversion esophagectomy, does not include
lymph node dissection of the mediastinum, and is only suitable for benign disease [27,28] . For all MIEs,
anastomotic techniques have varied. Cervical anastomosis has been described using either a two-layer
handsewn, circular or linear stapled anastomotic technique, with thoracic anastomosis employing the
same. More recently, the OrVil (Covidien, Mansfield, MA, USA), which is a stapling device utilized trans
orally, has touted benefits including the elimination of the technical assistance needed to attach the anvil
[27]
to the esophagus . Other technical issues include the decision of whether or not to perform a conduit
emptying procedure (pyloroplasty, pyloromyotomy, or Botox injection), gastric ischemic conditioning,
or prophylactic thoracic duct ligation, none of which have demonstrated a substantial difference in
[27]
outcomes .
Robotic esophagectomy
Although the use of minimally invasive techniques has many advantages including lower respiratory
complications and equivalent 30-day mortality [Table 2] [32-37] , the use of thoracoscopic and laparoscopic
surgery also has many drawbacks. First, visualization using both techniques is limited to two dimensions.
Second, with thoracoscopy, the need of the intercostal spaces to function as a fulcrum often leads to
[27]
nerve injury, postoperative pain, and paresthesias . As these obstacles have not been unique just to
esophagectomy, many have sought to bypass these challenges resulting in the development of robotic
surgery using computer-assisted surgical systems. Today, the da Vinci Surgical System (Intuitive Surgical,
Sunnyvale, CA, USA) [27,38] is the only robotic platform that is currently Food & Drug Administration
approved and available in a commercial platform. However, more models are on the horizon.
[38]
In 2002, Melvin et al. first reported completion of a robotic esophagectomy, and, in 2003, Horgan described
his experience performing the first robotic-assisted THE. Kernstine et al. [39,40] documented the first totally