Page 90 - Read Online
P. 90
Author Instructions
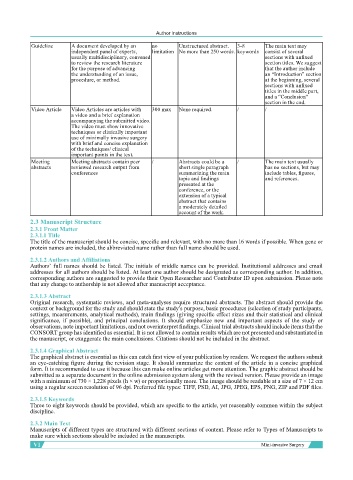
Guideline A document developed by an no Unstructured abstract. 3-8 The main text may
independent panel of experts, limitation No more than 250 words. keywords consist of several
usually multidisciplinary, convened sections with unfixed
to review the research literature section titles. We suggest
for the purpose of advancing that the author include
the understanding of an issue, an “Introduction” section
procedure, or method. at the beginning, several
sections with unfixed
titles in the middle part,
and a “Conclusion”
section in the end.
Video Article Video Articles are articles with 300 max None required. / /
a video and a brief explanation
accompanying the submitted video.
The video must show innovative
techniques or clinically important
use of minimally invasive surgery
with brief and concise explanation
of the techniques/ clinical
important points in the text.
Meeting Meeting abstracts contain peer / Abstracts could be a / The main text usually
abstracts reviewed research output from short single paragraph has no sections, but may
conferences summarizing the main include tables, figures,
topic and findings and references.
presented at the
conference, or the
extension of a typical
abstract that contains
a moderately detailed
account of the work.
2.3 Manuscript Structure
2.3.1 Front Matter
2.3.1.1 Title
The title of the manuscript should be concise, specific and relevant, with no more than 16 words if possible. When gene or
protein names are included, the abbreviated name rather than full name should be used.
2.3.1.2 Authors and Affiliations
Authors’ full names should be listed. The initials of middle names can be provided. Institutional addresses and email
addresses for all authors should be listed. At least one author should be designated as corresponding author. In addition,
corresponding authors are suggested to provide their Open Researcher and Contributor ID upon submission. Please note
that any change to authorship is not allowed after manuscript acceptance.
2.3.1.3 Abstract
Original research, systematic reviews, and meta-analyses require structured abstracts. The abstract should provide the
context or background for the study and should state the study’s purpose, basic procedures (selection of study participants,
settings, measurements, analytical methods), main findings (giving specific effect sizes and their statistical and clinical
significance, if possible), and principal conclusions. It should emphasize new and important aspects of the study or
observations, note important limitations, and not overinterpret findings. Clinical trial abstracts should include items that the
CONSORT group has identified as essential. It is not allowed to contain results which are not presented and substantiated in
the manuscript, or exaggerate the main conclusions. Citations should not be included in the abstract.
2.3.1.4 Graphical Abstract
The graphical abstract is essential as this can catch first view of your publication by readers. We request the authors submit
an eye-catching figure during the revision stage. It should summarize the content of the article in a concise graphical
form. It is recommended to use it because this can make online articles get more attention. The graphic abstract should be
submitted as a separate document in the online submission system along with the revised version. Please provide an image
with a minimum of 730 × 1,228 pixels (h × w) or proportionally more. The image should be readable at a size of 7 × 12 cm
using a regular screen resolution of 96 dpi. Preferred file types: TIFF, PSD, AI, JPG, JPEG, EPS, PNG, ZIP and PDF files.
2.3.1.5 Keywords
Three to eight keywords should be provided, which are specific to the article, yet reasonably common within the subject
discipline.
2.3.2 Main Text
Manuscripts of different types are structured with different sections of content. Please refer to Types of Manuscripts to
make sure which sections should be included in the manuscripts.
VI
Mini-invasive Surgery