Page 207 - Read Online
P. 207
Gholami et al. Mini-invasive Surg 2018;2:44 I http://dx.doi.org/10.20517/2574-1225.2018.44 Page 5 of 9
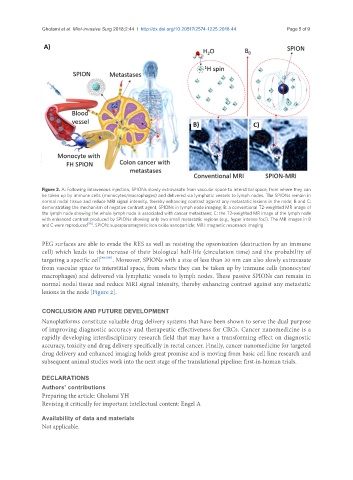
Figure 2. A: Following intravenous injection, SPIONs slowly extravasate from vascular space to interstitial space, from where they can
be taken up by immune cells (monocytes/macrophages) and delivered via lymphatic vessels to lymph nodes. The SPIONs remain in
normal nodal tissue and reduce MRI signal intensity, thereby enhancing contrast against any metastatic lesions in the node; B and C:
demonstrating the mechanism of negative contrast agent, SPIONs in lymph node imaging; B: a conventional T2-weighted MR image of
the lymph node showing the whole lymph node is associated with cancer metastases; C: the T2-weighted MR image of the lymph node
with enhanced contrast produced by SPIONs showing only two small metastatic regions (e.g., hyper intense foci). The MR images in B
and C were reproduced [96] . SPION: superparamagnetic iron oxide nanoparticle; MRI: magnetic resonance imaging
PEG surfaces are able to evade the RES as well as resisting the opsonisation (destruction by an immune
cell) which leads to the increase of their biological half-life (circulation time) and the probability of
targeting a specific cell [98-100] . Moreover, SPIONs with a size of less than 30 nm can also slowly extravasate
from vascular space to interstitial space, from where they can be taken up by immune cells (monocytes/
macrophages) and delivered via lymphatic vessels to lymph nodes. These passive SPIONs can remain in
normal nodal tissue and reduce MRI signal intensity, thereby enhancing contrast against any metastatic
lesions in the node [Figure 2].
CONCLUSION AND FUTURE DEVELOPMENT
Nanoplatforms constitute valuable drug delivery systems that have been shown to serve the dual purpose
of improving diagnostic accuracy and therapeutic effectiveness for CRCs. Cancer nanomedicine is a
rapidly developing interdisciplinary research field that may have a transforming effect on diagnostic
accuracy, toxicity and drug delivery specifically in rectal cancer. Finally, cancer nanomedicine for targeted
drug delivery and enhanced imaging holds great promise and is moving from basic cell line research and
subsequent animal studies work into the next stage of the translational pipeline: first-in-human trials.
DECLARATIONS
Authors’ contributions
Preparing the article: Gholami YH
Revising it critically for important intellectual content: Engel A
Availability of data and materials
Not applicable.