Page 36 - Read Online
P. 36
Okafor et al. Mini-invasive Surg 2024;8:28 https://dx.doi.org/10.20517/2574-1225.2023.128 Page 5 of 15
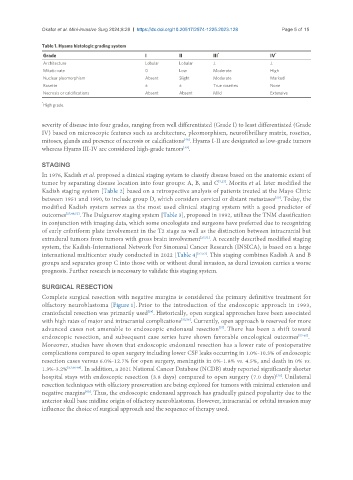
Table 1. Hyams histologic grading system
Grade I II III * IV *
Architecture Lobular Lobular ± ±
Mitotic rate 0 Low Moderate High
Nuclear pleomorphism Absent Slight Moderate Marked
Rosette ± ± True rosettes None
Necrosis or calcifications Absent Absent Mild Extensive
*
High grade.
severity of disease into four grades, ranging from well differentiated (Grade I) to least differentiated (Grade
IV) based on microscopic features such as architecture, pleomorphism, neurofibrillary matrix, rosettes,
mitoses, glands and presence of necrosis or calcifications . Hyams I-II are designated as low-grade tumors
[48]
[49]
whereas Hyams III-IV are considered high-grade tumors .
STAGING
In 1976, Kadish et al. proposed a clinical staging system to classify disease based on the anatomic extent of
tumor by separating disease location into four groups: A, B, and C [7,27] . Morita et al. later modified the
Kadish staging system [Table 2] based on a retrospective analysis of patients treated at the Mayo Clinic
between 1951 and 1990, to include group D, which considers cervical or distant metastases . Today, the
[50]
modified Kadish system serves as the most used clinical staging system with a good predictor of
outcomes [27,48,51] . The Dulguerov staging system [Table 3], proposed in 1992, utilizes the TNM classification
in conjunction with imaging data, which some oncologists and surgeons have preferred due to recognizing
of early cribriform plate involvement in the T2 stage as well as the distinction between intracranial but
extradural tumors from tumors with gross brain involvement [27,51] . A recently described modified staging
system, the Kadish-International Network For Sinonasal Cancer Research (INSICA), is based on a large
international multicenter study conducted in 2022 [Table 4] [52,53] . This staging combines Kadish A and B
groups and separates group C into those with or without dural invasion, as dural invasion carries a worse
prognosis. Further research is necessary to validate this staging system.
SURGICAL RESECTION
Complete surgical resection with negative margins is considered the primary definitive treatment for
olfactory neuroblastoma [Figure 1]. Prior to the introduction of the endoscopic approach in 1993,
craniofacial resection was primarily used . Historically, open surgical approaches have been associated
[54]
with high rates of major and intracranial complications [55,56] . Currently, open approach is reserved for more
advanced cases not amenable to endoscopic endonasal resection . There has been a shift toward
[57]
endoscopic resection, and subsequent case series have shown favorable oncological outcomes [57-65] .
Moreover, studies have shown that endoscopic endonasal resection has a lower rate of postoperative
complications compared to open surgery including lower CSF leaks occurring in 1.0%-10.3% of endoscopic
resection cases versus 6.0%-12.7% for open surgery, meningitis in 0%-1.8% vs. 4.5%, and death in 0% vs.
1.3%-3.2% [63,66-69] . In addition, a 2021 National Cancer Database (NCDB) study reported significantly shorter
hospital stays with endoscopic resection (3.8 days) compared to open surgery (7.0 days) . Unilateral
[70]
resection techniques with olfactory preservation are being explored for tumors with minimal extension and
[60]
negative margins . Thus, the endoscopic endonasal approach has gradually gained popularity due to the
anterior skull base midline origin of olfactory neuroblastoma. However, intracranial or orbital invasion may
influence the choice of surgical approach and the sequence of therapy used.