Page 60 - Read Online
P. 60
Hambright et al. Mini-invasive Surg 2024;8:19 https://dx.doi.org/10.20517/2574-1225.2024.08 Page 3 of 12
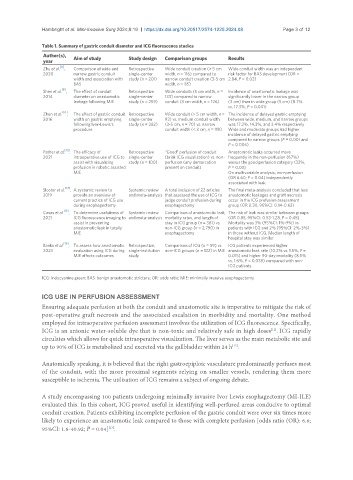
Table 1. Summary of gastric conduit diameter and ICG fluorescence studies
Author(s), Aim of study Study design Comparison groups Results
year
[8]
Zhu et al. , Comparison of wide and Retrospective Wide conduit creation (> 5 cm Wide conduit width was an independent
2020 narrow gastric conduit single-center width, n = 116) compared to risk factor for BAS development (OR =
width and association with study (n = 201) narrow conduit creation (3-5 cm 2.84, P = 0.02)
BAS width, n = 85)
[9]
Shen et al. , The effect of conduit Retrospective Wide conduits (5 cm width, n = Incidence of anastomotic leakage was
2014 diameter on anastomotic single-center 133) compared to narrow significantly lower in the narrow group
leakage following MIE study (n = 259) conduit (3 cm width, n = 126) (3 cm) than in wide group (5 cm) (8.7%
vs. 17.3%, P = 0.041)
Zhen et al. [10] , The effect of gastric conduit Retrospective Wide conduit (> 5 cm width, n = The incidence of delayed gastric emptying
2016 width on gastric emptying single-center 93) vs. medium conduit width between wide, medium, and narrow groups
following Ivor-Lewis’s study (n = 282) (3-5 cm, n = 70) vs. narrow was 17.2%, 14.3%, and 3.4% respectively
procedure conduit width (< 3 cm, n = 119) Wide and moderate groups had higher
incidence of delayed gastric emptying
compared to narrow groups (P = 0.001 and
P = 0.006)
[13]
Pather et al. , The efficacy of Retrospective “Good” perfusion of conduit Anastomotic leaks occurred more
2021 intraoperative use of ICG to single-center (brisk ICG visualization) vs. non- frequently in the non-perfusion (67%)
assist with visualizing study (n = 100) perfusion (any demarcation versus the good perfusion category (33%,
profusion in robotic assisted present on conduit) P = 0.03)
MIE On multivariable analysis, non-perfusion
(OR 6.60; P = 0.04) independently
associated with leak
[14]
Slooter et al. , A systemic review to Systemic review A total inclusion of 22 articles The final meta-analysis concluded that less
2019 provide an overview of and meta-analysis that assessed the use of ICG to anastomotic leakages and graft necrosis
current practice of ICG use judge conduit profusion during occur in the ICG prefusion assessment
during esophagectomy esophagectomy group (OR 0.30, 95%CI: 0.14-0.63)
[15]
Casas et al. , To determine usefulness of Systemic review Comparison of anastomotic leak, The risk of leak was similar between groups
2021 ICG fluorescence imaging to and meta-analysis mortality rates, and length of (OR 0.85, 95%CI: 0.53-1.28, P = 0.45)
assist in preventing stay in ICG group (n = 381) vs. Mortality was 3% (95%CI: 1%-9%) in
anastomotic leak in totally non-ICG group (n = 2,790) in patients with ICG and 2% (95%CI: 2%-3%)
MIE esophagectomy in those without ICG. Median length of
hospital stay was similar
Banks et al. [16] , To assess how anastomotic Retrospective, Comparison of ICG (n = 59) vs. ICG patients experienced higher
2023 evaluation using ICG during single-institution non-ICG groups (n = 122) in MIE anastomotic leak rate (10.2% vs. 1.6%, P =
MIE affects outcomes study 0.015) and higher 90-day mortality (8.5%
vs. 1.6%, P = 0.038) compared with non-
ICG patients
ICG: Indocyanine green; BAS: benign anastomotic stricture; OR: odds ratio; MIE: minimally invasive esophagectomy.
ICG USE IN PERFUSION ASSESSMENT
Ensuring adequate perfusion at both the conduit and anastomotic site is imperative to mitigate the risk of
post-operative graft necrosis and the associated escalation in morbidity and mortality. One method
employed for intraoperative perfusion assessment involves the utilization of ICG fluorescence. Specifically,
ICG is an anionic water-soluble dye that is non-toxic and relatively safe in high doses . ICG rapidly
[11]
circulates which allows for quick intraoperative visualization. The liver serves as the main metabolic site and
up to 90% of ICG is metabolized and excreted via the gallbladder within 24 h .
[12]
Anatomically speaking, it is believed that the right gastroepiploic vasculature predominantly perfuses most
of the conduit, with the more proximal segments relying on smaller vessels, rendering them more
susceptible to ischemia. The utilization of ICG remains a subject of ongoing debate.
A study encompassing 100 patients undergoing minimally invasive Ivor Lewis esophagectomy (MI-ILE)
evaluated this. In this cohort, ICG proved useful in identifying well-perfused areas conducive to optimal
conduit creation. Patients exhibiting incomplete perfusion of the gastric conduit were over six times more
likely to experience an anastomotic leak compared to those with complete perfusion [odds ratio (OR): 6.6;
95%CI: 1.6-40.92; P = 0.04] .
[13]