Page 131 - Read Online
P. 131
Page 383 Santiago et al. J Transl Genet Genom 2021;5:380-95 https://dx.doi.org/10.20517/jtgg.2021.16
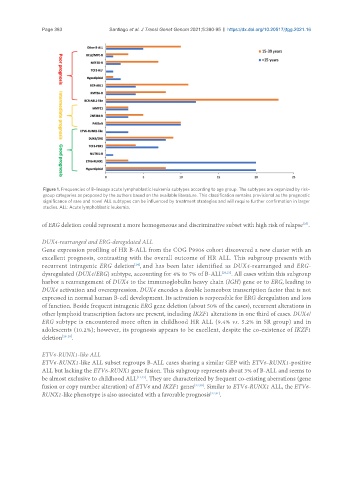
Figure 1. Frequencies of B-lineage acute lymphoblastic leukemia subtypes according to age group. The subtypes are organized by risk-
group categories as proposed by the authors based on the available literature. This classification remains provisional as the prognostic
significance of rare and novel ALL subtypes can be influenced by treatment strategies and will require further confirmation in larger
studies. ALL: Acute lymphoblastic leukemia.
of ERG deletion could represent a more homogeneous and discriminative subset with high risk of relapse .
[25]
DUX4-rearranged and ERG-deregulated ALL
Gene expression profiling of HR B-ALL from the COG P9906 cohort discovered a new cluster with an
excellent prognosis, contrasting with the overall outcome of HR ALL. This subgroup presents with
recurrent intragenic ERG deletion , and has been later identified as DUX4-rearranged and ERG-
[14]
dysregulated (DUX4/ERG) subtype, accounting for 4% to 7% of B-ALL [26,27] . All cases within this subgroup
harbor a rearrangement of DUX4 to the immunoglobulin heavy chain (IGH) gene or to ERG, leading to
DUX4 activation and overexpression. DUX4 encodes a double homeobox transcription factor that is not
expressed in normal human B-cell development. Its activation is responsible for ERG deregulation and loss
of function. Beside frequent intragenic ERG gene deletion (about 50% of the cases), recurrent alterations in
other lymphoid transcription factors are present, including IKZF1 alterations in one third of cases. DUX4/
ERG subtype is encountered more often in childhood HR ALL (9.4% vs. 5.2% in SR group) and in
adolescents (10.2%); however, its prognosis appears to be excellent, despite the co-existence of IKZF1
deletion [26-29] .
ETV6-RUNX1-like ALL
ETV6-RUNX1-like ALL subset regroups B-ALL cases sharing a similar GEP with ETV6-RUNX1-positive
ALL but lacking the ETV6-RUNX1 gene fusion. This subgroup represents about 3% of B-ALL and seems to
be almost exclusive to childhood ALL [11,27] . They are characterized by frequent co-existing aberrations (gene
fusion or copy number alteration) of ETV6 and IKZF1 genes [27,30] . Similar to ETV6-RUNX1 ALL, the ETV6-
RUNX1-like phenotype is also associated with a favorable prognosis [27,31] .