Page 137 - Read Online
P. 137
Page 288 Balasubramaniam et al. J Transl Genet Genom 2020;4:285-306 I http://dx.doi.org/10.20517/jtgg.2020.34
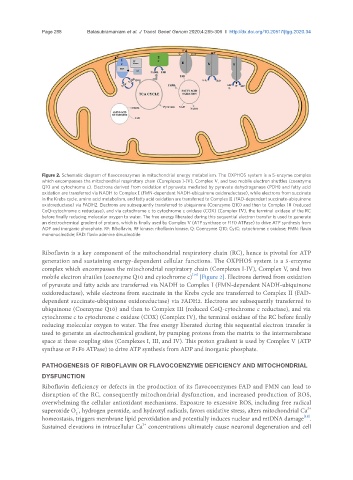
Figure 2. Schematic diagram of flavocoenzymes in mitochondrial energy metabolism. The OXPHOS system is a 5-enzyme complex
which encompasses the mitochondrial respiratory chain (Complexes I-IV), Complex V, and two mobile electron shuttles (coenzyme
Q10 and cytochrome c). Electrons derived from oxidation of pyruvate mediated by pyruvate dehydrogenase (PDH) and fatty acid
oxidation are transferred via NADH to Complex I (FMN-dependent NADH-ubiquinone oxidoreductase), while electrons from succinate
in the Krebs cycle, amino acid metabolism, and fatty acid oxidation are transferred to Complex II (FAD-dependent succinate-ubiquinone
oxidoreductase) via FADH2. Electrons are subsequently transferred to ubiquinone (Coenzyme Q10) and then to Complex III (reduced
CoQ-cytochrome c reductase), and via cytochrome c to cytochrome c oxidase (COX) (Complex IV), the terminal oxidase of the RC
before finally reducing molecular oxygen to water. The free energy liberated during this sequential electron transfer is used to generate
an electrochemical gradient of protons, which is finally used by Complex V (ATP synthase or F1F0 ATPase) to drive ATP synthesis from
ADP and inorganic phosphate. RF: Riboflavin; RF kinase: riboflavin kinase; Q: Coenzyme Q10; CytC: cytochrome c oxidase; FMN: flavin
mononucleotide; FAD: flavin adenine dinucleotide
Riboflavin is a key component of the mitochondrial respiratory chain (RC), hence is pivotal for ATP
generation and sustaining energy-dependent cellular functions. The OXPHOS system is a 5-enzyme
complex which encompasses the mitochondrial respiratory chain (Complexes I-IV), Complex V, and two
[14]
mobile electron shuttles (coenzyme Q10 and cytochrome c) [Figure 2]. Electrons derived from oxidation
of pyruvate and fatty acids are transferred via NADH to Complex I (FMN-dependent NADH-ubiquinone
oxidoreductase), while electrons from succinate in the Krebs cycle are transferred to Complex II (FAD-
dependent succinate-ubiquinone oxidoreductase) via FADH2. Electrons are subsequently transferred to
ubiquinone (Coenzyme Q10) and then to Complex III (reduced CoQ-cytochrome c reductase), and via
cytochrome c to cytochrome c oxidase (COX) (Complex IV), the terminal oxidase of the RC before finally
reducing molecular oxygen to water. The free energy liberated during this sequential electron transfer is
used to generate an electrochemical gradient, by pumping protons from the matrix to the intermembrane
space at three coupling sites (Complexes I, III, and IV). This proton gradient is used by Complex V (ATP
synthase or F1F0 ATPase) to drive ATP synthesis from ADP and inorganic phosphate.
PATHOGENESIS OF RIBOFLAVIN OR FLAVOCOENZYME DEFICIENCY AND MITOCHONDRIAL
DYSFUNCTION
Riboflavin deficiency or defects in the production of its flavocoenzymes FAD and FMN can lead to
disruption of the RC, consequently mitochondrial dysfunction, and increased production of ROS,
overwhelming the cellular antioxidant mechanisms. Exposure to excessive ROS, including free radical
-
2+
superoxide O , hydrogen peroxide, and hydroxyl radicals, favors oxidative stress, alters mitochondrial Ca
2
[15]
homeostasis, triggers membrane lipid peroxidation and potentially induces nuclear and mtDNA damage .
Sustained elevations in intracellular Ca concentrations ultimately cause neuronal degeneration and cell
2+