Page 18 - Read Online
P. 18
Page 343 Giliberti et al. J Transl Genet Genom 2024;8:340-54 https://dx.doi.org/10.20517/jtgg.2024.41
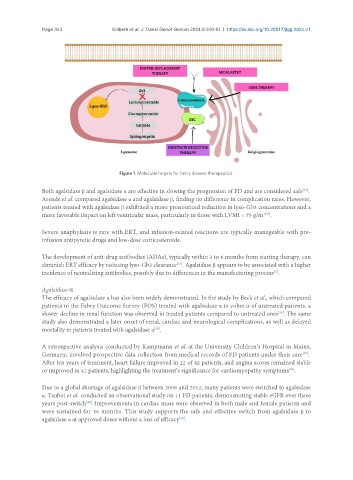
Figure 1. Molecular targets for Fabry disease therapeutics.
Both agalsidase β and agalsidase α are effective in slowing the progression of FD and are considered safe .
[35]
Arends et al. compared agalsidase α and agalsidase β, finding no difference in complication rates. However,
patients treated with agalsidase β exhibited a more pronounced reduction in lyso-Gb3 concentrations and a
2[36]
more favorable impact on left ventricular mass, particularly in those with LVMI < 75 g/m .
Severe anaphylaxis is rare with ERT, and infusion-related reactions are typically manageable with pre-
infusion antipyretic drugs and low-dose corticosteroids.
The development of anti-drug antibodies (ADAs), typically within 3 to 6 months from starting therapy, can
diminish ERT efficacy by reducing lyso-Gb3 clearance . Agalsidase β appears to be associated with a higher
[37]
[7]
incidence of neutralizing antibodies, possibly due to differences in the manufacturing process .
Agalsidase α
The efficacy of agalsidase α has also been widely demonstrated. In the study by Beck et al., which compared
patients in the Fabry Outcome Survey (FOS) treated with agalsidase α to cohorts of untreated patients, a
slower decline in renal function was observed in treated patients compared to untreated ones . The same
[38]
study also demonstrated a later onset of renal, cardiac and neurological complications, as well as delayed
mortality in patients treated with agalsidase α .
[38]
A retrospective analysis conducted by Kampmann et al. at the University Children’s Hospital in Mainz,
Germany, involved prospective data collection from medical records of FD patients under their care .
[39]
After ten years of treatment, heart failure improved in 22 of 42 patients, and angina scores remained stable
or improved in 41 patients, highlighting the treatment’s significance for cardiomyopathy symptoms .
[39]
Due to a global shortage of agalsidase β between 2009 and 2012, many patients were switched to agalsidase
α. Tsuboi et al. conducted an observational study on 11 FD patients, demonstrating stable eGFR over three
years post-switch . Improvements in cardiac mass were observed in both male and female patients and
[40]
were sustained for 36 months. This study supports the safe and effective switch from agalsidase β to
agalsidase α at approved doses without a loss of efficacy .
[40]