Page 9 - Read Online
P. 9
Mejia et al. J Transl Genet Genom 2024;8:216-24 https://dx.doi.org/10.20517/jtgg.2024.11 Page 220
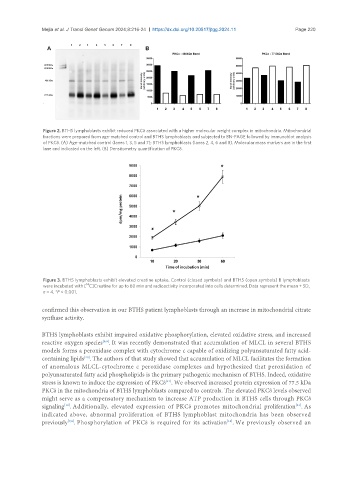
Figure 2. BTHS lymphoblasts exhibit reduced PKCδ associated with a higher molecular weight complex in mitochondria. Mitochondrial
fractions were prepared from age-matched control and BTHS lymphoblasts and subjected to BN-PAGE followed by immunoblot analysis
of PKCδ. (A) Age-matched control (lanes 1, 3, 5 and 7); BTHS lymphoblasts (lanes 2, 4, 6 and 8). Molecular mass markers are in the first
lane and indicated on the left. (B) Densitometry quantification of PKCδ.
Figure 3. BTHS lymphoblasts exhibit elevated creatine uptake. Control (closed symbols) and BTHS (open symbols) B lymphoblasts
14
were incubated with [ C]Creatine for up to 60 min and radioactivity incorporated into cells determined. Data represent the mean + SD,
n = 4. *P < 0.001.
confirmed this observation in our BTHS patient lymphoblasts through an increase in mitochondrial citrate
synthase activity.
BTHS lymphoblasts exhibit impaired oxidative phosphorylation, elevated oxidative stress, and increased
reactive oxygen species . It was recently demonstrated that accumulation of MLCL in several BTHS
[8,9]
models forms a peroxidase complex with cytochrome c capable of oxidizing polyunsaturated fatty acid-
containing lipids . The authors of that study showed that accumulation of MLCL facilitates the formation
[20]
of anomalous MLCL-cytochrome c peroxidase complexes and hypothesized that peroxidation of
polyunsaturated fatty acid phospholipids is the primary pathogenic mechanism of BTHS. Indeed, oxidative
stress is known to induce the expression of PKCδ . We observed increased protein expression of 77.5 kDa
[21]
PKCδ in the mitochondria of BTHS lymphoblasts compared to controls. The elevated PKCδ levels observed
might serve as a compensatory mechanism to increase ATP production in BTHS cells through PKCδ
signaling . Additionally, elevated expression of PKCδ promotes mitochondrial proliferation . As
[21]
[12]
indicated above, abnormal proliferation of BTHS lymphoblast mitochondria has been observed
[13]
previously . Phosphorylation of PKCδ is required for its activation . We previously observed an
[7,8]