Page 51 - Read Online
P. 51
Barwell et al. J Transl Genet Genom 2018;2:13. I https://doi.org/10.20517/jtgg.2018.17 Page 5 of 10
[5]
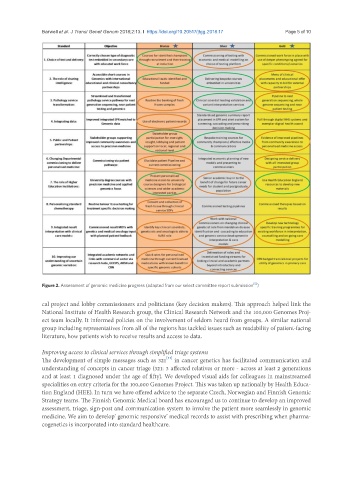
Figure 2. Assessment of genomic medicine progress (adapted from our select committee report submission )
cal project and lobby commissioners and politicians (key decision makers). This approach helped link the
National Institute of Health Research group, the Clinical Research Network and the 100,000 Genomes Proj-
ect team locally. It informed policies on the involvement of seldom heard from groups. A similar national
group including representatives from all of the regions has tackled issues such as readability of patient-facing
literature, how patients wish to receive results and access to data.
Improving access to clinical services through simplified triage systems
[11]
The development of simple messages such as 321 in cancer genetics has facilitated communication and
understanding of concepts in cancer triage (321: 3 affected relatives or more - across at least 2 generations
and at least 1 diagnosed under the age of fifty). We developed visual aids for colleagues in mainstreamed
specialities on entry criteria for the 100,000 Genomes Project. This was taken up nationally by Health Educa-
tion England (HEE). In turn we have offered advice to the separate Czech, Norwegian and Finnish Genomic
Strategy teams. The Finnish Genomic Medical board has encouraged us to continue to develop an improved
assessment, triage, sign-post and communication system to involve the patient more seamlessly in genomic
medicine. We aim to develop’ genomic responsive’ medical records to assist with prescribing when pharma-
cogenetics is incorporated into standard healthcare.