Page 7 - Read Online
P. 7
Bhasin et al. J Transl Genet Genom 2024;8:55-76 https://dx.doi.org/10.20517/jtgg.2023.46 Page 57
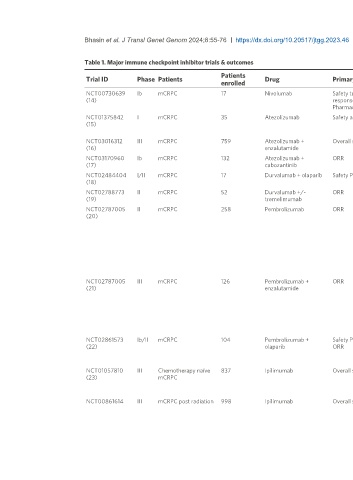
Table 1. Major immune checkpoint inhibitor trials & outcomes
Patients
Trial ID Phase Patients Drug Primary endpoint Outcome
enrolled
NCT00730639 Ib mCRPC 17 Nivolumab Safety tumor Grade 3/4 TRAE 14%
(14) response ORR 0.0%
Pharmacokinetics
NCT01375842 I mCRPC 35 Atezolizumab Safety activity Grade 3/4 TRAE 11.4%
(15) Confirmed PSA 50 8.6%
Median OS 14.7 (95%CI: 5.9-NE) months
NCT03016312 III mCRPC 759 Atezolizumab + Overall survival Median OS 15.2 months (95%CI: 14-17) in atezolizumab + enzalutamide arm vs. OS 16.6
(16) enzalutamide (95%CI: 14.7-18.4) months in enzalutamide alone
NCT03170960 Ib mCRPC 132 Atezolizumab + ORR ORR 23%(95%CI: 17%-32%)
(17) cabozantinib Grade 3/4 TRAE 55%
NCT02484404 I/II mCRPC 17 Durvalumab + olaparib Safety PFS Grade 3/4 TRAE 12% 12-month PFS 51.5% (95%CI: 25.7%-72.3%)
(18)
NCT02788773 II mCRPC 52 Durvalumab +/- ORR ORR in durvalumab alone: 0% (95%CI: 0%-25%)
(19) tremelimumab ORR in durvalumab + tremelimumab: 16% (95%CI: 6%-32%)
NCT02787005 II mCRPC 258 Pembrolizumab ORR Cohort 1:
(20) - ORR 5% (95%CI: 2%-11%)
- Median OS 9.5 months
Cohort 2:
- ORR 3% (95%CI: < 1%-11%)
- Median OS 7.9 months
Cohort 3:
- ORR < 1%
- Median OS 14.1 months
- Grade 3-5 TRAE 15%
NCT02787005 III mCRPC 126 Pembrolizumab + ORR Cohort 4:
(21) enzalutamide - ORR 12.3% (95%CI: 6.1%-21.5%)
- Median OS 17.6 months (95%CI: 14-22.6)
- Grade 3/4 TRAE 27.2%
Cohort 5:
- ORR NA
- Median OS 20.8 months (95%CI: 14.1-28.9)
- Grade 3/4 TRAE 28.9%
NCT02861573 Ib/II mCRPC 104 Pembrolizumab + Safety PSA response PSA response 15%
(22) olaparib ORR ORR 8.5% (95%CI: 2.8%-19%)
Median OS 14 months (95%CI: 10.4-18.2)
Grade 3-5 TRAE 48%
NCT01057810 III Chemotherapy naïve 837 Ipilimumab Overall survival Median OS 28.7 months (95%CI: 24.5-32.5) vs. placebo 29.7 months (95%CI: 26.1-34.2)
(23) mCRPC Median PFS 5.6 months ipilimumab vs. 3.8 months placebo
PSA response rate 23% ipilimumab vs. 8% placebo
Grade 3/4 Immune-related TRAE 31% ipilimumab vs. 2% placebo
NCT00861614 III mCRPC post radiation 998 Ipilimumab Overall survival Median OS 11.2 months (95%CI: 9.5-12.7) vs. 10 months (placebo) (95%CI: 8.3-11)