Page 32 - Read Online
P. 32
Page 122 Bibi et al. J Transl Genet Genom 2024;8:119-161 https://dx.doi.org/10.20517/jtgg.2023.50
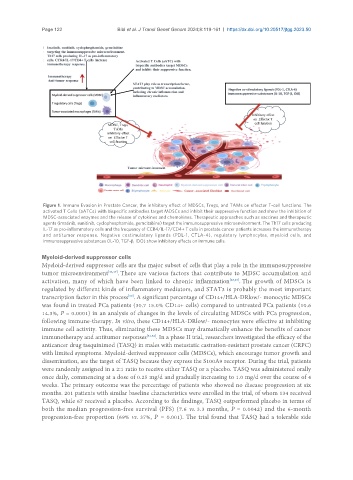
Figure 1. Immune Evasion in Prostate Cancer, the inhibitory effect of MDSCs, Tregs, and TAMs on effector T-cell functions. The
activated T Cells (aATCs) with bispecific antibodies target MDSCs and inhibit their suppressive function and show the inhibition of
MDSC-associated enzymes and the release of cytokines and chemokines. Therapeutic approaches such as vaccines and therapeutic
agents (imatinib, sunitinib, cyclophosphamide, gemcitabine) target the immunosuppressive microenvironment. The Th17 cells producing
IL-17 as pro-inflammatory cells and the frequency of CCR4/IL-17/CD4+ T cells in prostate cancer patients increases the immunotherapy
and antitumor response. Negative costimulatory ligands (PDL-1, CTLA-4), regulatory lymphocytes, myeloid cells, and
immunosuppressive substances (IL-10, TGF-β, IDO) show inhibitory effects on immune cells.
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells are the major subset of cells that play a role in the immunosuppressive
tumor microenvironment [46,47] . There are various factors that contribute to MDSC accumulation and
activation, many of which have been linked to chronic inflammation [48,49] . The growth of MDSCs is
regulated by different kinds of inflammatory mediators, and STAT3 is probably the most important
[50]
transcription factor in this process . A significant percentage of CD14+/HLA-DRlow/- monocytic MDSCs
was found in treated PCa patients (30.7 15.0% CD14+ cells) compared to untreated PCa patients (10.6
14.3%, P = 0.0001) in an analysis of changes in the levels of circulating MDSCs with PCa progression,
following immune-therapy. In vitro, these CD14+/HLA-DRlow/- monocytes were effective at inhibiting
immune cell activity. Thus, eliminating these MDSCs may dramatically enhance the benefits of cancer
immunotherapy and antitumor responses [51,52] . In a phase II trial, researchers investigated the efficacy of the
anticancer drug tasquinimod (TASQ) in males with metastatic castration-resistant prostate cancer (CRPC)
with limited symptoms. Myeloid-derived suppressor cells (MDSCs), which encourage tumor growth and
dissemination, are the target of TASQ because they express the S100A9 receptor. During the trial, patients
were randomly assigned in a 2:1 ratio to receive either TASQ or a placebo. TASQ was administered orally
once daily, commencing at a dose of 0.25 mg/d and gradually increasing to 1.0 mg/d over the course of 4
weeks. The primary outcome was the percentage of patients who showed no disease progression at six
months. 201 patients with similar baseline characteristics were enrolled in the trial, of whom 134 received
TASQ, while 67 received a placebo. According to the findings, TASQ outperformed placebo in terms of
both the median progression-free survival (PFS) (7.6 vs. 3.3 months, P = 0.0042) and the 6-month
progression-free proportion (69% vs. 37%, P = 0.001). The trial found that TASQ had a tolerable side