Page 574 - Read Online
P. 574
Page 10 of 19 Cordover et al. J Cancer Metastasis Treat 2020;6:45 I http://dx.doi.org/10.20517/2394-4722.2020.101
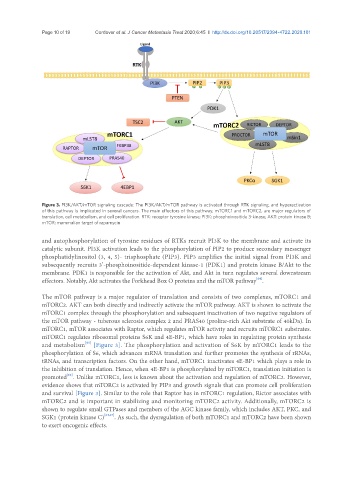
Figure 3. PI3K/AKT/mTOR signaling cascade: The PI3K/AKT/mTOR pathway is activated through RTK signaling, and hyperactivation
of this pathway is implicated in several cancers. The main effectors of this pathway, mTORC1 and mTORC2, are major regulators of
translation, cell metabolism, and cell proliferation. RTK: receptor tyrosine kinase; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B;
mTOR: mammalian target of rapamycin
and autophosphorylation of tyrosine residues of RTKs recruit PI3K to the membrane and activate its
catalytic subunit. PI3K activation leads to the phosphorylation of PIP2 to produce secondary messenger
phosphatidylinositol (3, 4, 5)- trisphosphate (PIP3). PIP3 amplifies the initial signal from PI3K and
subsequently recruits 3’-phosphoinositide-dependent kinase-1 (PDK1) and protein kinase B/Akt to the
membrane. PDK1 is responsible for the activation of Akt, and Akt in turn regulates several downstream
[64]
effectors. Notably, Akt activates the Forkhead Box O proteins and the mTOR pathway .
The mTOR pathway is a major regulator of translation and consists of two complexes, mTORC1 and
mTORC2. AKT can both directly and indirectly activate the mTOR pathway. AKT is shown to activate the
mTORC1 complex through the phosphorylation and subsequent inactivation of two negative regulators of
the mTOR pathway - tuberous sclerosis complex 2 and PRAS40 (proline-rich Akt substrate of 40kDa). In
mTORC1, mTOR associates with Raptor, which regulates mTOR activity and recruits mTORC1 substrates.
mTORC1 regulates ribosomal proteins S6K and 4E-BP1, which have roles in regulating protein synthesis
[65]
and metabolism [Figure 3]. The phosphorylation and activation of S6K by mTORC1 leads to the
phosphorylation of S6, which advances mRNA translation and further promotes the synthesis of rRNAs,
tRNAs, and transcription factors. On the other hand, mTORC1 inactivates 4E-BP1 which plays a role in
the inhibition of translation. Hence, when 4E-BP1 is phosphorylated by mTORC1, translation initiation is
[66]
promoted . Unlike mTORC1, less is known about the activation and regulation of mTORC2. However,
evidence shows that mTORC2 is activated by PIP3 and growth signals that can promote cell proliferation
and survival [Figure 3]. Similar to the role that Raptor has in mTORC1 regulation, Rictor associates with
mTORC2 and is important in stabilizing and monitoring mTORC2 activity. Additionally, mTORC2 is
shown to regulate small GTPases and members of the AGC kinase family, which includes AKT, PKC, and
SGK1 (protein kinase C) [64,67] . As such, the dysregulation of both mTORC1 and mTORC2 have been shown
to exert oncogenic effects.