Page 161 - Read Online
P. 161
Ioannides et al. J Cancer Metastasis Treat 2020;6:15 I http://dx.doi.org/10.20517/2394-4722.2020.22 Page 5 of 7
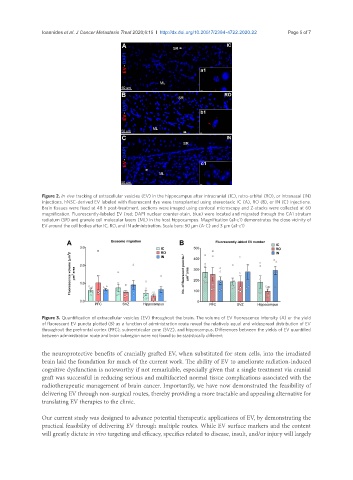
Figure 2. In vivo tracking of extracellular vesicles (EV) in the hippocampus after intracranial (IC), retro-orbital (RO), or intranasal (IN)
injections. hNSC-derived EV labeled with fluorescent dye were transplanted using stereotaxic IC (A), RO (B), or IN (C) injections.
Brain tissues were fixed at 48 h post-treatment, sections were imaged using confocal microscopy and Z-stacks were collected at 60
magnification. Fluorescently-labeled EV (red; DAPI nuclear counter-stain, blue) were located and migrated through the CA1 stratum
radiatum (SR) and granule cell molecular layers (ML) in the host hippocampus. Magnification (a1-c1) demonstrates the close vicinity of
EV around the cell bodies after IC, RO, and IN administration. Scale bars: 50 µm (A-C) and 3 µm (a1-c1)
Figure 3. Quantification of extracellular vesicles (EV) throughout the brain. The volume of EV fluorescence intensity (A) or the yield
of fluorescent EV puncta plotted (B) as a function of administration route reveal the relatively equal and widespread distribution of EV
throughout the prefrontal cortex (PFC), subventricular zone (SVZ), and hippocampus. Differences between the yields of EV quantified
between administration route and brain subregion were not found to be statistically different
the neuroprotective benefits of cranially grafted EV, when substituted for stem cells, into the irradiated
brain laid the foundation for much of the current work. The ability of EV to ameliorate radiation-induced
cognitive dysfunction is noteworthy if not remarkable, especially given that a single treatment via cranial
graft was successful in reducing serious and multifaceted normal tissue complications associated with the
radiotherapeutic management of brain cancer. Importantly, we have now demonstrated the feasibility of
delivering EV through non-surgical routes, thereby providing a more tractable and appealing alternative for
translating EV therapies to the clinic.
Our current study was designed to advance potential therapeutic applications of EV, by demonstrating the
practical feasibility of delivering EV through multiple routes. While EV surface markers and the content
will greatly dictate in vivo targeting and efficacy, specifics related to disease, insult, and/or injury will largely