Page 847 - Read Online
P. 847
Page 6 of 8 Burches et al. J Cancer Metastasis Treat 2019;5:63 I http://dx.doi.org/10.20517/2394-4722.2019.012
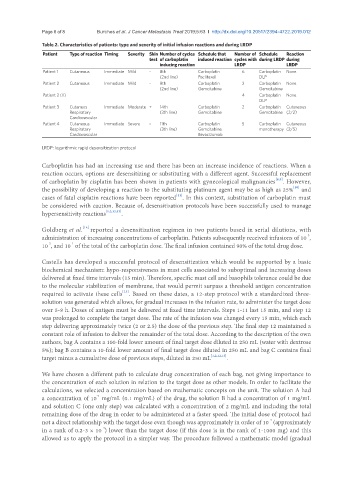
Table 2. Characteristics of patients: type and severity of initial infusion reactions and during LRDP
Patient Type of reaction Timing Severity Skin Number of cycles Schedule that Number of Schedule Reaction
test of carboplatin induced reaction cycles with during LRDP during
inducing reaction LRDP LRDP
Patient 1 Cutaneous Immediate Mild - 8th Carboplatin 6 Carboplatin None
(2nd line) Paclitaxel DLP
Patient 2 Cutaneous Immediate Mild - 9th Carboplatin 2 Carboplatin None
(2nd line) Gemcitabine Gemcitabine
Patient 2 (II) 4 Carboplatin None
DLP
Patient 3 Cutaneos Immediate Moderate + 14th Carboplatin 2 Carboplatin Cutaneous
Respiratory (3th line) Gemcitabine Gemcitabine (2/2)
Cardiovascular
Patient 4 Cutaneous Immediate Severe - 11th Carboplatin 5 Carboplatin Cutaneous
Respiratory (3th line) Gemcitabine monotherapy (2/5)
Cardiovascular Bevacizumab
LRDP: logarithmic rapid desensitization protocol
Carboplatin has had an increasing use and there has been an increase incidence of reactions. When a
reaction occurs, options are desensitizing or substituting with a different agent. Successful replacement
[8,9]
of carboplatin by cisplatin has been shown in patients with gynecological malignancies . However,
the possibility of developing a reaction to the substituting platinum agent may be as high as 25% and
[10]
[11]
cases of fatal cisplatin reactions have been reported . In this context, substitution of carboplatin must
be considered with caution. Because of, desensitization protocols have been successfully used to manage
hypersensitivity reactions [1,2,12,13] .
Goldberg et al. [14] reported a desensitization regimen in two patients based in serial dilutions, with
administration of increasing concentrations of carboplatin. Patients subsequently received infusions of 10 ,
-3
-1
-2
10 , and 10 of the total of the carboplatin dose. The final infusion contained 90% of the total drug dose.
Castells has developed a successful protocol of desensitization which would be supported by a basic
biochemical mechanism: hypo-responsiveness in mast cells associated to suboptimal and increasing doses
delivered at fixed time intervals (15 min). Therefore, specific mast cell and basophils tolerance could be due
to the molecular stabilization of membrane, that would permit surpass a threshold antigen concentration
[15]
required to activate these cells . Based on these dates, a 12-step protocol with a standardized three-
solution was generated which allows, for gradual increases in the infusion rate, to administer the target dose
over 5-8 h. Doses of antigen must be delivered at fixed time intervals. Steps 1-11 last 15 min, and step 12
was prolonged to complete the target dose. The rate of the infusion was changed every 15 min, which each
step delivering approximately twice (2 or 2.5) the dose of the previous step. The final step 12 maintained a
constant role of infusion to deliver the remainder of the total dose. According to the description of the own
authors, bag A contains a 100-fold lower amount of final target dose diluted in 250 mL (water with dextrose
5%); bag B contains a 10-fold lower amount of final target dose diluted in 250 mL and bag C contains final
target minus a cumulative dose of previous steps, diluted in 250 mL [1,2,12,13] .
We have chosen a different path to calculate drug concentration of each bag, not giving importance to
the concentration of each solution in relation to the target dose as other models. In order to facilitate the
calculations, we selected a concentration based on mathematic concepts on the unit. The solution A had
-1
a concentration of 10 mg/mL (0.1 mg/mL) of the drug, the solution B had a concentration of 1 mg/mL
and solution C (one only step) was calculated with a concentration of 2 mg/mL and including the total
remaining dose of the drug in order to be administered at a faster speed. The initial dose of protocol had
-4
not a direct relationship with the target dose even though was approximately in order of 10 (approximately
-4
in a rank of 0.2-3 × 10 ) lower than the target dose (if this dose is in the rank of 1-1000 mg) and this
allowed us to apply the protocol in a simpler way. The procedure followed a mathematic model (gradual