Page 846 - Read Online
P. 846
Burches et al. J Cancer Metastasis Treat 2019;5:63 I http://dx.doi.org/10.20517/2394-4722.2019.012 Page 5 of 8
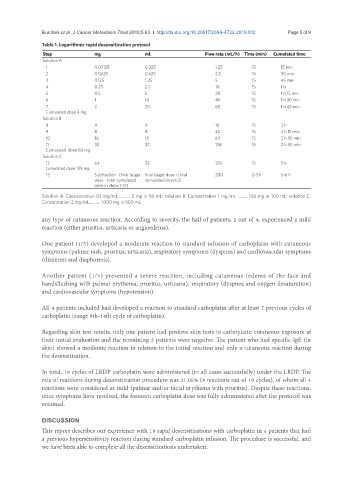
Table 1. Logarithmic rapid desensitization protocol
Step mg mL Flow rate (mL/h) Time (min) Cumulated time
Solution A
1 0.03125 0.325 1,25 15 15 min
2 0.0625 0.625 2.5 15 30 min
3 0.125 1.25 5 15 45 min
4 0.25 2.5 10 15 1 h
5 0.5 5 20 15 1 h 15 min
6 1 10 40 15 1 h 30 min
7 2 20 80 15 1 h 45 min
Cumulated dose 4 mg
Solution B
8 4 4 16 15 2 h
9 8 8 32 15 2 h 15 min
10 16 16 64 15 2 h 30 min
11 32 32 128 15 2 h 45 min
Cumulated dose 60 mg
Solution C
12 64 32 128 15 3 h
cumulated dose 128 mg
13 Subtraction (final target final target dose - total 200 2-3 h 5-6 h
dose - total cumulated cumulated dose)/2
dose in steps 1-12)
Solution A: Concentration 0.1 mg/mL .......... 5 mg in 50 mL; solution B: Concentration 1 mg/mL ......... 100 mg in 100 mL; solution C:
Concentration 2 mg/mL .......... 1000 mg in 500 mL
any type of cutaneous reaction. According to severity, the half of patients, 2 out of 4, experienced a mild
reaction (either pruritus, urticaria or angioedema).
One patient (1/5) developed a moderate reaction to standard infusion of carboplatin with cutaneous
symptoms (palmar rash, pruritus, urticaria), respiratory symptoms (dyspnea) and cardiovascular symptoms
(dizziness and diaphoresis).
Another patient (1/5) presented a severe reaction, including cutaneous (edema of the face and
handsflushing with palmar erythema, pruritus, urticaria), respiratory (dyspnea and oxygen desaturation)
and cardiovascular symptoms (hypotension).
All 4 patients included had developed a reaction to standard carboplatin after at least 7 previous cycles of
carboplatin (range 8th-14th cycle of carboplatin).
Regarding skin test results, only one patient had positive skin tests to carboplatin cutaneous exposure at
their initial evaluation and the remaining 3 patients were negative. The patient who had specific-IgE (in
skin) showed a moderate reaction in relation to the initial reaction and only a cutaneous reaction during
the desensitization.
In total, 19 cycles of LRDP carboplatin were administered (in all cases successfully) under the LRDP. The
rate of reactions during desensitization procedure was 21.05% (4 reactions out of 19 cycles), of whom all 4
reactions were considered as mild (palmar and/or facial erythema with pruritus). Despite these reactions,
once symptoms have resolved, the foreseen carboplatin dose was fully administered after the protocol was
resumed.
DISCUSSION
This report describes our experience with 19 rapid desensitizations with carboplatin in 4 patients that had
a previous hypersensitivity reaction during standard carboplatin infusion. The procedure is successful, and
we have been able to complete all the desensitizations undertaken.