Page 617 - Read Online
P. 617
Peyvandi et al. J Cancer Metastasis Treat 2019;5:44 I http://dx.doi.org/10.20517/2394-4722.2019.16 Page 5 of 24
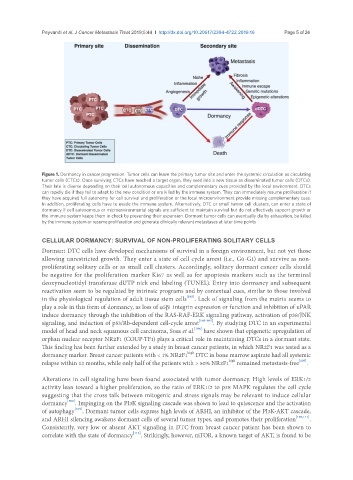
Figure 1. Dormancy in cancer progression. Tumor cells can leave the primary tumor site and enter the systemic circulation as circulating
tumor cells (CTCs). Once surviving CTCs have reached a target organ, they seed into a new tissue as disseminated tumor cells (DTCs).
Their fate is diverse depending on their cell autonomous capacities and complementary cues provided by the local environment. DTCs
can rapidly die if they fail to adapt to the new condition or are killed by the immune system. They can immediately resume proliferation if
they have acquired full autonomy for cell survival and proliferation or the local microenvironment provide missing complementary cues.
In addition, proliferating cells have to evade the immune system. Alternatively, DTC or small tumor cell clusters, can enter a state of
dormancy if cell autonomous or microenvironmental signals are sufficient to maintain survival but do not effectively support growth or
the immune system keeps them in check by preventing their expansion. Dormant tumor cells can eventually die by exhaustion, be killed
by the immune system or resume proliferation and generate clinically relevant metastases at later time points
CELLULAR DORMANCY: SURVIVAL OF NON-PROLIFERATING SOLITARY CELLS
Dormant DTC cells have developed mechanisms of survival in a foreign environment, but not yet those
allowing unrestricted growth. They enter a state of cell cycle arrest (i.e., G0-G1) and survive as non-
proliferating solitary cells or as small cell clusters. Accordingly, solitary dormant cancer cells should
be negative for the proliferation marker Ki67 as well as for apoptosis markers such as the terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Entry into dormancy and subsequent
reactivation seem to be regulated by intrinsic programs and by contextual cues, similar to those involved
in the physiological regulation of adult tissue stem cells [103] . Lack of signaling from the matrix seems to
play a role in this form of dormancy, as loss of α5β1 integrin expression or function and inhibition of uPAR
induce dormancy through the inhibition of the RAS-RAF-ERK signaling pathway, activation of p38/JNK
signaling, and induction of p53/Rb-dependent cell-cycle arrest [103-105] . By studying DTC in an experimental
[106]
model of head and neck squamous cell carcinoma, Sosa et al. have shown that epigenetic upregulation of
orphan nuclear receptor NR2F1 (COUP-TF1) plays a critical role in maintaining DTCs in a dormant state.
This finding has been further extended by a study in breast cancer patients, in which NR2F1 was tested as a
high
dormancy marker. Breast cancer patients with < 1% NR2F1 DTC in bone marrow aspirate had all systemic
relapse within 12 months, while only half of the patients with > 50% NR2F1 remained metastasis-free [107] .
high
Alterations in cell signaling have been found associated with tumor dormancy. High levels of ERK1/2
activity lean toward a higher proliferation, so the ratio of ERK1/2 to p38 MAPK regulates the cell cycle
suggesting that the cross talk between mitogenic and stress signals may be relevant to induce cellular
dormancy [108] . Impinging on the PI3K signaling cascade was shown to lead to quiescence and the activation
of autophagy [109] . Dormant tumor cells express high levels of ARHI, an inhibitor of the PI3K-AKT cascade,
and ARHI silencing awakens dormant cells of several tumor types, and promotes their proliferation [110,111] .
Consistently, very low or absent AKT signaling in DTC from breast cancer patient has been shown to
correlate with the state of dormancy [112] . Strikingly, however, mTOR, a known target of AKT, is found to be