Page 338 - Read Online
P. 338
Mizejewski. J Cancer Metastasis Treat 2018;4:27 I http://dx.doi.org/10.20517/2394-4722.2018.20 Page 5 of 7
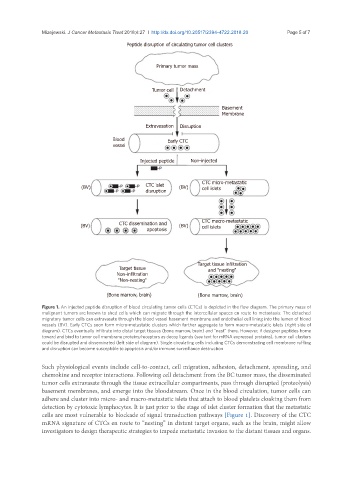
Peptide disruption of circulating tumor cell clusters
Primary tumor mass
Tumor cell Detachment
Basement
Membrane
Extravasation Disruption
Blood Early CTC
vessel
Injected peptide Non-injected
(BV) CTC islet (BV) CTC micro-metastatic
cell islets
disruption
CTC macro-metastatic
(BV) CTC dissemination and (BV) cell islets
apoptosis
Target tissue infiltration
Target tissue and "nesting"
Non-infiltration
"Non-nesting"
(Bone marrow, brain) (Bone marrow, brain)
Figure 1. An injected peptide disruption of blood circulating tumor cells (CTCs) is depicted in the flow diagram. The primary mass of
malignant tumors are known to shed cells which can migrate through the intercellular spaces en route to metastasis. The detached
migratory tumor cells can extravasate through the blood vessel basement membrane and endothelial cell lining into the lumen of blood
vessels (BV). Early CTCs soon form micro-metastatic clusters which further aggregate to form macro-metastatic islets (right side of
diagram). CTCs eventually infiltrate into distal target tissues (bone marrow, brain) and “nest” there. However, if designer peptides home
toward and bind to tumor cell membrane proteins/receptors as decoy ligands (see text for mRNA expressed proteins), tumor cell clusters
could be disrupted and disseminated (left side of diagram). Single circulating cells including CTCs demonstrating cell membrane ruffling
and disruption can become susceptible to apoptosis and/or immune surveillance destruction
Such physiological events include cell-to-contact, cell migration, adhesion, detachment, spreading, and
chemokine and receptor interactions. Following cell detachment from the BC tumor mass, the disseminated
tumor cells extravasate through the tissue extracellular compartments, pass through disrupted (proteolysis)
basement membranes, and emerge into the bloodstream. Once in the blood circulation, tumor cells can
adhere and cluster into micro- and macro-metastatic islets that attach to blood platelets cloaking them from
detection by cytotoxic lymphocytes. It is just prior to the stage of islet cluster formation that the metastatic
cells are most vulnerable to blockade of signal transduction pathways [Figure 1]. Discovery of the CTC
mRNA signature of CTCs en route to “nesting” in distant target organs, such as the brain, might allow
investigators to design therapeutic strategies to impede metastatic invasion to the distant tissues and organs.