Page 192 - Read Online
P. 192
Elzaafarany et al. J Cancer Metastasis Treat 2018;4:14 I http://dx.doi.org/10.20517/2394-4722.2017.55 Page 5 of 8
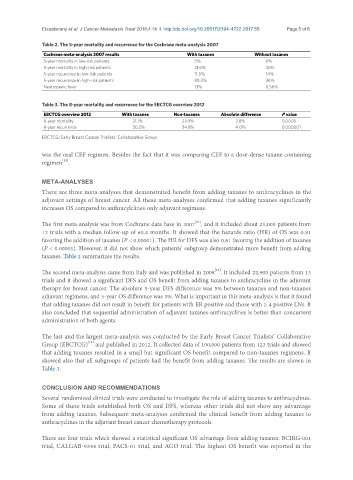
Table 2. The 5-year mortality and recurrence for the Cochrane meta-analysis 2007
Cochrane meta-analysis 2007 results With taxanes Without taxanes
5-year mortality in low-risk patients 5% 6%
5-year mortality in high-risk patients 21.6% 26%
5-year recurrence in low-risk patients 11.5% 14%
5-year recurrence in high-risk patients 30.3% 36%
Neutropenic fever 13% 0.56%
Table 3. The 8-year mortality and recurrence for the EBCTCG overview 2012
EBCTCG overview 2012 With taxanes Non-taxanes Absolute difference P value
8-year mortality 21.1% 23.9% 2.8% 0.0005
8-year recurrence 30.2% 34.8% 4.0% 0.000001
EBCTCG: Early Breast Cancer Trialists’ Collaborative Group
was the oral CEF regimen. Besides the fact that it was comparing CEF to a dose-dense taxane containing
[12]
regimen .
META-ANALYSES
There are three meta-analyses that demonstrated benefit from adding taxanes to anthracyclines in the
adjuvant settings of breast cancer. All these meta-analyses confirmed that adding taxanes significantly
increases OS compared to anthracylclines-only adjuvant regimens.
[13]
The first meta-analysis was from Cochrane data base in 2007 , and it included about 21,000 patients from
12 trials with a median follow-up of 60.4 months. It showed that the hazards ratio (HR) of OS was 0.81
favoring the addition of taxanes (P < 0.00001). The HR for DFS was also 0.81 favoring the addition of taxanes
(P < 0.00001). However, it did not show which patients’ subgroup demonstrated more benefit from adding
taxanes. Table 2 summarizes the results.
[14]
The second meta-analysis came from Italy and was published in 2008 . It included 22,900 patients from 13
trials and it showed a significant DFS and OS benefit from adding taxanes to anthracycline in the adjuvant
therapy for breast cancer. The absolute 5-year DFS difference was 5% between taxanes and non-taxanes
adjuvant regimens, and 5-year OS difference was 3%. What is important in this meta-analysis is that it found
that adding taxanes did not result in benefit for patients with ER positive and those with ≥ 4 positive LNs. It
also concluded that sequential administration of adjuvant taxanes-anthracyclines is better than concurrent
administration of both agents.
The last and the largest meta-analysis was conducted by the Early Breast Cancer Trialists’ Collaborative
[15]
Group (EBCTCG) and published in 2012. It collected data of 100,000 patients from 123 trials and showed
that adding taxanes resulted in a small but significant OS benefit compared to non-taxanes regimens. It
showed also that all subgroups of patients had the benefit from adding taxanes. The results are shown in
Table 3.
CONCLUSION AND RECOMMENDATIONS
Several randomized clinical trials were conducted to investigate the role of adding taxanes to anthracyclines.
Some of these trials established both OS and DFS, whereas other trials did not show any advantage
from adding taxanes. Subsequent meta-analyses confirmed the clinical benefit from adding taxanes to
anthracyclines in the adjuvant breast cancer chemotherapy protocols.
There are four trials which showed a statistical significant OS advantage from adding taxanes: BCIRG-001
trial, CALGAB-9344 trial, PACS-01 trial, and AGO trial. The highest OS benefit was reported in the