Page 189 - Read Online
P. 189
Page 2 of 8 Elzaafarany et al. J Cancer Metastasis Treat 2018;4:14 I http://dx.doi.org/10.20517/2394-4722.2017.55
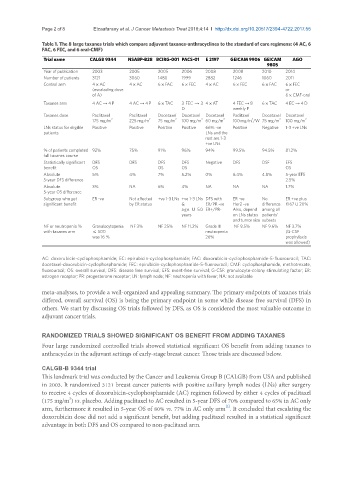
Table 1. The 8 large taxanes trials which compare adjuvant taxanes-anthracyclines to the standard of care regimens: (4 AC, 6
FAC, 6 FEC, and 6 oral-CMF)
Trial name CALGB 9344 NSABP-B28 BCIRG-001 PACS-01 E 2197 GEICAM 9906 GEICAM AGO
9805
Year of publication 2003 2005 2005 2006 2008 2008 2010 2014
Number of patients 3121 3060 1480 1999 2882 1246 1060 2011
Control arm 4 × AC 4 × AC 6 × FAC 6 × FEC 4 × AC 6 × FEC 6 × FAC 6 × FEC
(escalading dose or
of A) 6 × CMF-oral
Taxanes arm 4 AC → 4 P 4 AC → 4 P 6 × TAC 3 FEC → 3 4 × AT 4 FEC → 8 6 × TAC 4 EC → 4 D
D weekly P
Taxanes dose Paclitaxel Paclitaxel Docetaxel Docetaxel Docetaxel Paclitaxel Docetaxel Docetaxel
2
2
175 mg/m 2 225 mg/m 2 75 mg/m 2 100 mg/m 60 mg/m 2 100 mg/m /W 75 mg/m 2 100 mg/m 2
LNs status for eligible Positive Positive Positive Positive 66% -ve Positive Negative 1-3 +ve LNs
patients LNs and the
rest are 1-3
+ve LNs
% of patients completed 92% 75% 91% 96% 94% 99.5% 94.5% 81.2%
full taxanes course
Statistically significant DFS DFS DFS DFS Negative DFS DSF EFS
benefit OS OS OS OS
Absolute 5% 4% 7% 5.2% 0% 6.4% 4.8% 5-year EFS
5-year DFS difference 2.5%
Absolute 3% NA 6% 4% NA NA NA 1.7%
5-year OS difference
Subgroup who get ER -ve Not affected +ve 1-3 LNs +ve 1-3 LNs DFS with ER -ve No ER +ve plus
significant benefit by ER status & ER/PR -ve Her2 -ve difference KI67 ≥ 20%
age ≥ 50 ER+/PR- Also, depend among all
years on LNs status patients’
and tumor size subsets
NF or neutropenia % Granulocytopenia NF 3% NF 25% NF 11.2% Grade III NF 9.5% NF 9.6% NF 3.7%
with taxanes arm ≤ 500 neutropenia (G-CSF
was 16 % 26% prophylaxis
was allowed)
AC: doxorubicin-cyclophosphamide; EC: epirubicin-cyclophosphamide; FAC: doxorubicin-cyclophosphamide-5-fluorouracil; TAC:
docetaxel-doxorubicin-cyclophosphamide; FEC: epirubicin-cyclophosphamide-5-fluorouracil; CMF: cyclophosphamide, methotrexate,
fluorouracil; OS: overall survival; DFS: disease free survival; EFS: event-free survival; G-CSF: granulocyte-colony stimulating factor; ER:
estrogen receptor; PR: progesterone receptor; LN: lymph node; NF: neutropenia with fever; NA: not available
meta-analyses, to provide a well-organized and appealing summary. The primary endpoints of taxanes trials
differed, overall survival (OS) is being the primary endpoint in some while disease free survival (DFS) in
others. We start by discussing OS trials followed by DFS, as OS is considered the most valuable outcome in
adjuvant cancer trials.
RANDOMIZED TRIALS SHOWED SIGNIFICANT OS BENEFIT FROM ADDING TAXANES
Four large randomized controlled trials showed statistical significant OS benefit from adding taxanes to
anthracycles in the adjuvant settings of early-stage breast cancer. Those trials are discussed below.
CALGB-B 9344 trial
This landmark trial was conducted by the Cancer and Leukemia Group B (CALGB) from USA and published
in 2003. It randomized 3121 breast cancer patients with positive axillary lymph nodes (LNs) after surgery
to receive 4 cycles of doxorubicin-cyclophosphamide (AC) regimen followed by either 4 cycles of paclitaxel
2
(175 mg/m ) vs. placebo. Adding paclitaxel to AC resulted in 5-year DFS of 70% compared to 65% in AC only
arm, furthermore it resulted in 5-year OS of 80% vs. 77% in AC only arm . It concluded that escalating the
[2]
doxorubicin dose did not add a significant benefit, but adding paclitaxel resulted in a statistical significant
advantage in both DFS and OS compared to non-paclitaxel arm.