Page 419 - Read Online
P. 419
Cerna et al. Nanodrugs in brain tumors
Table 2: FDA-approved anticancer nanodrugs. Modified from [80]
Name Description Indication Approval (year)
DaunoXome Liposomal daunorubicin HIV-releated Kaposi sa FDA 96
DepoCyt Liposomal cytarabin Lymphomatous meningitis FDA 96
Oncaspar PEG asparaginase Acute lymphoblastic leukemia FDA 94
Abraxane Albumin-bound paclitaxel nanospheres Various cancers FDA 05 EMEA 08, FDA 13
Pancreatic ca
Myocet Liposomal doxorubicin Breast ca Europe + Canada
Marqibo Liposomal vincristin Acute lymphoblastic leukemia FDA 12
Genexol Paclitaxel loaded polymeric micelle Breast ca, small cell lung ca Europe + Korea
Onivyde Liposomal irinotecan Pancreatic ca FDA 15
sa: sarcoma; ca: carcinoma
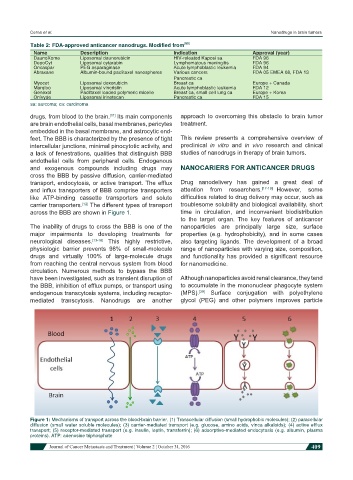
drugs, from blood to the brain. Its main components approach to overcoming this obstacle to brain tumor
[11]
are brain endothelial cells, basal membranes, pericytes treatment.
embedded in the basal membrane, and astrocytic end-
feet. The BBB is characterized by the presence of tight This review presents a comprehensive overview of
intercellular junctions, minimal pinocytotic activity, and preclinical in vitro and in vivo research and clinical
a lack of fenestrations, qualities that distinguish BBB studies of nanodrugs in therapy of brain tumors.
endothelial cells from peripheral cells. Endogenous
and exogenous compounds including drugs may NANOCARIERS FOR ANTICANCER DRUGS
cross the BBB by passive diffusion, carrier-mediated
transport, endocytosis, or active transport. The efflux Drug nanodelivery has gained a great deal of
and influx transporters of BBB comprise transporters attention from researchers. [17-19] However, some
like ATP-binding cassette transporters and solute difficulties related to drug delivery may occur, such as
carrier transporters. The different types of transport troublesome solubility and biological availability, short
[12]
across the BBB are shown in Figure 1. time in circulation, and inconvenient biodistribution
to the target organ. The key features of anticancer
The inability of drugs to cross the BBB is one of the nanoparticles are principally large size, surface
major impairments to developing treatments for properties (e.g. hydrophobicity), and in some cases
neurological diseases. [13-16] This highly restrictive, also targeting ligands. The development of a broad
physiologic barrier prevents 98% of small-molecule range of nanoparticles with varying size, composition,
drugs and virtually 100% of large-molecule drugs and functionality has provided a significant resource
from reaching the central nervous system from blood for nanomedicine.
circulation. Numerous methods to bypass the BBB
have been investigated, such as transient disruption of Although nanoparticles avoid renal clearance, they tend
the BBB, inhibition of efflux pumps, or transport using to accumulate in the mononuclear phagocyte system
endogenous transcytosis systems, including receptor- (MPS). Surface conjugation with polyethylene
[20]
mediated transcytosis. Nanodrugs are another glycol (PEG) and other polymers improves particle
Figure 1: Mechanisms of transport across the blood-brain barrier. (1) Transcellular diffusion (small hydrophobic molecules); (2) paracellular
diffusion (small water soluble molecules); (3) carrier-mediated transport (e.g. glucose, amino acids, vinca alkaloids); (4) active efflux
transport; (5) receptor-mediated transport (e.g. insulin, leptin, transferrin); (6) adsorptive-mediated endocytosis (e.g. albumin, plasma
proteins). ATP: adenosine triphosphate
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ October 31, 2016 409