Page 201 - Read Online
P. 201
Table 3: Adverse events
Grade 1 Grade 2 Grade 3 Grade 4
Patient number Patient number Patient number Patient number
(%) (%) (%) (%)
Heamatological toxicity 2 (7) 2 (7) 3 (10) 0
Neutropenia
Aneamia 2 (7) 1 (3) 2 (7) 0
Thrombocytopenia 1 (3) 0 0 0
Non heamatological toxicity 2 (7) 1 (3) 1 (3) 0
Impaired liver enzymes
Fever 2 (7) 0 0 0
Nausea/vomiting 3 (10) 1 (3) 0 0
Anorexia 2 (7) 0 0 0
Diarrhea 2 (7) 1 (3) 0 0
Fatigue 3 (10) 2 (7) 0 0
Convulsion 2 (7) 0 0 0
Headache 4 (13) 0 0 0
Insomnia 1(3) 0 0 0
Alopecia 9 (30) 4 (13) 0 0
Otitis externa 1 (3) 0 0 0
Scalp dermatitis 2 (7) 0 0 0
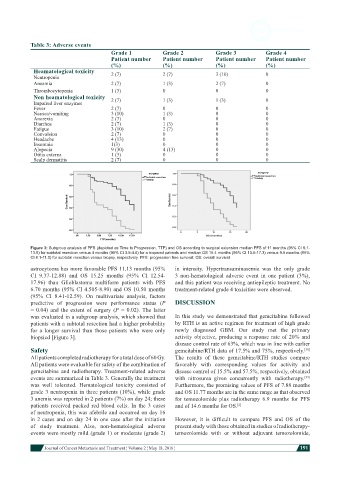
Figure 3: Subgroup analysis of PFS (depicted as Time to Progression, TTP) and OS according to surgical extension median PFS of 11 months (95% CI 8.1-
13.9) for subtotal resection versus 4 months (95% CI 3.5-4.6) for a biopsied patients and median OS 15.4 months (95% CI 13.5-17.3) versus 9.5 months (95%
CI 8.1-11.0) for subtotal resection versus biopsy, respectively. PFS: progression free survival; OS: overall survival
astrocytoma has more favorable PFS 11.13 months (95% in intensity. Hypertransaminasemia was the only grade
CI 9.37-12.88) and OS 15.25 months (95% CI 12.54- 3 non-hematological adverse event in one patient (3%),
17.96) than Glioblastoma multiform patients with PFS and this patient was receiving antiepileptic treatment. No
6.70 months (95% CI 4.505-8.90) and OS 10.50 months treatment-related grade 4 toxicities were observed.
(95% CI 8.41-12.59). On multivariate analysis, factors
predictive of progression were performance status (P DISCUSSION
= 0.04) and the extent of surgery (P = 0.02). The latter
was evaluated in a subgroup analysis, which showed that In this study we demonstrated that gemcitabine followed
patients with a subtotal resection had a higher probability by RTH is an active regimen for treatment of high grade
for a longer survival than those patients who were only newly diagnosed GBM. Our study met the primary
biopsied [Figure 3]. activity objective, producing a response rate of 20% and
disease control rate of 63%, which was in line with earlier
Safety gemcitabine/RTH data of 17.5% and 75%, respectively.
[30]
All patients completed radiotherapy for a total dose of 60 Gy. The results of these gemcitabine/RTH studies compare
All patients were evaluable for safety of the combination of favorably with corresponding values for activity and
gemcitabine and radiotherapy. Treatment-related adverse disease control of 15.5% and 57.5%, respectively, obtained
events are summarized in Table 3. Generally the treatment with nitrosurea given concurrently with radiotherapy.
[34]
was well tolerated. Hematological toxicity consisted of Furthermore, the promising values of PFS of 7.88 months
grade 3 neutropenia in three patients (10%), while grade and OS 11.77 months are in the same range as that observed
3 anemia was reported in 2 patients (7%) on day 24; these for temozolomide plus radiotherapy 6.9 months for PFS
patients received packed red blood cells. In the 3 cases and of 14.6 months for OS. [2]
of neutropenia, this was afebrile and occurred on day 16
in 2 cases and on day 24 in one case after the initiation However, it is difficult to compare PFS and OS of the
of study treatment. Also, non-hematological adverse present study with those obtained in studies of radiotherapy-
events were mostly mild (grade 1) or moderate (grade 2) temozolomide with or without adjuvant temozolomide,
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ May 18, 2016 ¦ 191