Page 66 - Read Online
P. 66
Page 4 of 9 Rosenzweig et al. J Cancer Metastasis Treat 2022;8:46 https://dx.doi.org/10.20517/2394-4722.2022.58
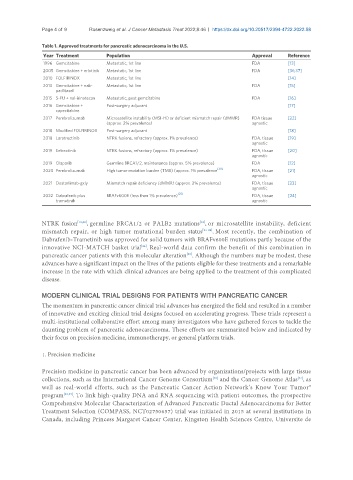
Table 1. Approved treatments for pancreatic adenocarcinoma in the U.S.
Year Treatment Population Approval Reference
1996 Gemcitabine Metastatic, 1st line FDA [13]
2005 Gemcitabine + erlotinib Metastatic, 1st line FDA [36,37]
2010 FOLFIRINOX Metastatic, 1st line [14]
2013 Gemcitabine + nab- Metastatic, 1st line FDA [15]
paclitaxel
2015 5-FU + nal-irinotecan Metastatic, post gemcitabine FDA [16]
2016 Gemcitabine + Post-surgery adjuvant [17]
capecitabine
2017 Pembrolizumab Microsatellite instability (MSI-Hi) or deficient mismatch repair (dMMR) FDA tissue [22]
(approx. 2% prevalence) agnostic
2018 Modified FOLFIRINOX Post-surgery adjuvant [18]
2018 Larotrectinib NTRK fusions, refractory (approx. 1% prevalence) FDA, tissue [19]
agnostic
2019 Entrectinib NTRK fusions, refractory (approx. 1% prevalence) FDA, tissue [20]
agnostic
2019 Olaparib Germline BRCA1/2, maintenance (approx. 5% prevalence) FDA [12]
2020 Pembrolizumab High tumor mutation burden (TMB) (approx. 1% prevalence [38] FDA, tissue [21]
agnostic
2021 Dostarlimab-gxly Mismatch repair deficiency (dMMR) (approx. 2% prevalence) FDA, tissue [23]
agnostic
2022 Dabrafenib plus BRAFv600E (less than 1% prevalence) [25] FDA, tissue [24]
trametinib agnostic
[12]
NTRK fusion [19,20] , germline BRCA1/2 or PALB2 mutations , or microsatellite instability, deficient
mismatch repair, or high tumor mutational burden status [21-23] . Most recently, the combination of
Dabrafenib-Trametinib was approved for solid tumors with BRAFv600E mutations partly because of the
[24]
innovative NCI-MATCH basket trial . Real-world data confirm the benefit of this combination in
pancreatic cancer patients with this molecular alteration . Although the numbers may be modest, these
[25]
advances have a significant impact on the lives of the patients eligible for these treatments and a remarkable
increase in the rate with which clinical advances are being applied to the treatment of this complicated
disease.
MODERN CLINICAL TRIAL DESIGNS FOR PATIENTS WITH PANCREATIC CANCER
The momentum in pancreatic cancer clinical trial advances has energized the field and resulted in a number
of innovative and exciting clinical trial designs focused on accelerating progress. These trials represent a
multi-institutional collaborative effort among many investigators who have gathered forces to tackle the
daunting problem of pancreatic adenocarcinoma. These efforts are summarized below and indicated by
their focus on precision medicine, immunotherapy, or general platform trials.
1. Precision medicine
Precision medicine in pancreatic cancer has been advanced by organizations/projects with large tissue
[26]
[27]
collections, such as the International Cancer Genome Consortium and the Cancer Genome Atlas , as
well as real-world efforts, such as the Pancreatic Cancer Action Network’s Know Your Tumor®
program [28,29] . To link high-quality DNA and RNA sequencing with patient outcomes, the prospective
Comprehensive Molecular Characterization of Advanced Pancreatic Ductal Adenocarcinoma for Better
Treatment Selection (COMPASS, NCT02750657) trial was initiated in 2015 at several institutions in
Canada, including Princess Margaret Cancer Center, Kingston Health Sciences Centre, Universite de