Page 70 - Read Online
P. 70
RTH was applied to the gross tumor volume with 2-3 cm Table 1: Patient characteristics
margin for the clinical target volume. Radiotherapy was Total patients 30
carried out using linear accelerator with 6-15 MV photons. Age in years, median (range) 52 (30-69)
Gemcitabine was administered at a fixed dose of 175 mg/m² Gender
by intravenous infusion starting 24 h prior to radiotherapy Male 22 (73%)
in the first week and then once weekly before RTH for the Female 8 (27%)
whole duration of the radiotherapy. Toxicities were graded ECOG performance status, 1 (0-2)
using the NCIC-CTG expanded common toxicity criteria. median (range)
Evaluation during protocol treatment included history and Pathology
physical examinations (including full clinical neurologic Anaplastic Astrocytoma 8 (27%)
examination), biochemical profiles; and imaging studies. Glioblastoma multiforma 22 (73%)
Contrast-enhanced (gadolinium-DTPA) MRI of the brain Surgical procedure
was uniformly adopted for tumor assessment and evaluation Subtotal resection 10 (33%)
of response. Baseline MRI examination was performed Biopsy 20 (67%)
24-48 h after surgery and then within 1 week prior to the ECOG: Eastern Cooperative Oncology Group
start of the experimental treatment, 4 weeks after the end
of chemo-radiotherapy and every 8 weeks thereafter until Table 2: Treatment response
evidence of disease progression. Toxicity assessments were Response Patient number (%)
done weekly during the radiotherapy and then one month
from the end of the treatment then every 2 months or when Complete response (CR) 1 (3)
clinically indicated. Toxicity was graded according to NCI- Partial response (PR) 5 (17)
CTC version 3.0. Response was assessed using standard Stable disease (SD) 13(43)
[32]
Macdonald criteria, but patients were not considered Progressive disease (PD) 11 (37)
[33]
to have had complete or partial responses unless clinical Disease control rate (CR+PR+SD) 19/30 (63)
neurologic assessment was improved or stable. Patients Tumor response rate (CR+PR) 6/30 (20)
were monitored until death.
Statistics
The duration of response was calculated from the first day
of treatment to the date of progression for patients who
achieved complete or partial response. Progression-free
survival, analyzed by Kaplan-Meier method including 95%
CI, was defined as the period of time elapsed from the first
day of treatment to the date of disease progression, relapse
or death from any cause. Overall survival was defined as
the interval from the first day of study treatment to the date
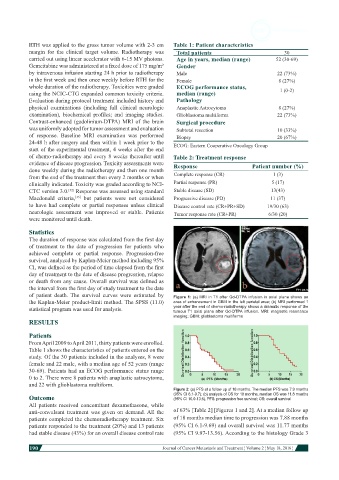
of patient death. The survival curves were estimated by Figure 1: (a) MRI in T1 after Gd-DTPA infusion in axial plane shows an
the Kaplan-Meier product-limit method. The SPSS (11.0) area of enhancement in GBM in the left parietal area; (b) MRI performed 1
statistical program was used for analysis. year after the end of chemo-radiotherapy shows a dramatic response of the
tumour T1 axial plane after Gd-DTPA infusion. MRI: magnetic resonance
imaging; GBM: glioblastoma multiforme
RESULTS
Patients
From April 2009 to April 2011, thirty patients were enrolled.
Table 1 shows the characteristics of patients entered on the
study. Of the 30 patients included in the analyses, 8 were
female and 22 male, with a median age of 52 years (range
30-69). Patients had an ECOG performance status range
0 to 2. There were 8 patients with anaplastic astrocytoma,
and 22 with glioblastoma multiform.
Figure 2: (a) PFS at a follow up of 18 months. The median PFS was 7.9 months
Outcome (95% CI 6.1-9.7); (b) analysis of OS for 18 months, median OS was 11.8 months
(95% CI 10.0-13.6). PFS: progression free survival; OS: overall survival
All patients received concomitant dexamethasone, while
anti-convulsant treatment was given on demand. All the of 63% [Table 2] [Figures 1 and 2]. At a median follow up
patients completed the chemoradiotherapy treatment. Six of 18 months median time to progression was 7.88 months
patients responded to the treatment (20%) and 13 patients (95% CI 6.1-9.69) and overall survival was 11.77 months
had stable disease (43%) for an overall disease control rate (95% CI 9.97-13.56). According to the histology Grade 3
190
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ May 18, 2016 ¦