Page 85 - Read Online
P. 85
Page 6 of 12 Bongiovanni et al. J Cancer Metastasis Treat 2022;8:44 https://dx.doi.org/10.20517/2394-4722.2022.78
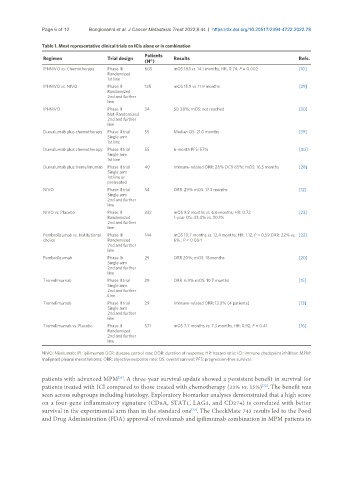
Table 1. Most representative clinical trials on ICIs alone or in combination
Patients
Regimen Trial design Results Refs.
(N°)
IPI-NIVO vs. Chemotherapy Phase III 605 mOS 18.1 vs. 14.1 months; HR, 0.74; P = 0.002 [10]
Randomized
1st line
IPI-NIVO vs. NIVO Phase II 125 mOS 15.9 vs. 11.9 months [29]
Randomized
2nd and further
line
IPI-NIVO Phase II 34 SD 38%; mOS: not reached [30]
Not-Randomized
2nd and further
line
Durvalumab plus chemotherapy Phase II trial 55 Median OS: 21.0 months [39]
Single arm
1st line
Durvalumab plus chemotherapy Phase II trial 55 6-month PFS: 57% [40]
Single arm
1st line
Durvalumab plus tremelimumab Phase II trial 40 Immune-related ORR: 28% DCR 65%; mOS: 16.5 months [28]
Single arm
1st line or
pretreated
NIVO Phase II trial 34 ORR: 29% mOS: 17.3 months [12]
Single arm
2nd and further
line
NIVO vs. Placebo Phase II 332 mOS 9.2 months vs. 6.6 months; HR: 0.72 [23]
Randomized 1-year OS: 43.4% vs. 30.1%
2nd and further
line
Pembrolizumab vs. Institutional Phase III 144 mOS 10.7 months vs. 12.4 months; HR: 1.12; P = 0.59 ORR: 22% vs. [22]
choice Randomized 6% ; P = 0.004
2nd and further
line
Pembrolizumab Phase Ib 25 ORR 20%; mOS: 18 months [20]
Single arm
2nd and further
line
Tremelimumab Phase II trial 29 ORR: 6.9% mOS: 10.7 months [15]
Single arm
2nd and further
Line
Tremelimumab Phase II trial 29 Immune-related ORR: 13.8% (4 patients) [13]
Single arm
2nd and further
line
Tremelimumab vs. Placebo Phase II 571 mOS 7.7 months vs. 7.3 months; HR: 0.92; P = 0.41 [16]
Randomized
2nd and further
line
NIVO: Nivolumab; IPI: ipilimumab DCR: disease control rate; DOR: duration of response; HR: hazard ratio; ICI: immune checkpoint inhibitor; MPM:
malignant pleural mesothelioma; ORR: objective response rate; OS: overall survival; PFS: progression-free survival.
patients with advanced MPM . A three-year survival update showed a persistent benefit in survival for
[31]
[32]
patients treated with ICI compared to those treated with chemotherapy (23% vs. 15%) . The benefit was
seen across subgroups including histology. Exploratory biomarker analyses demonstrated that a high score
on a four-gene inflammatory signature (CD8A, STAT1, LAG3, and CD274) is correlated with better
[32]
survival in the experimental arm than in the standard one . The CheckMate 743 results led to the Food
and Drug Administration (FDA) approval of nivolumab and ipilimumab combination in MPM patients in