Page 66 - Read Online
P. 66
Lue et al. J Cancer Metastasis Treat 2022;8:11 https://dx.doi.org/10.20517/2394-4722.2021.193 Page 9 of 25
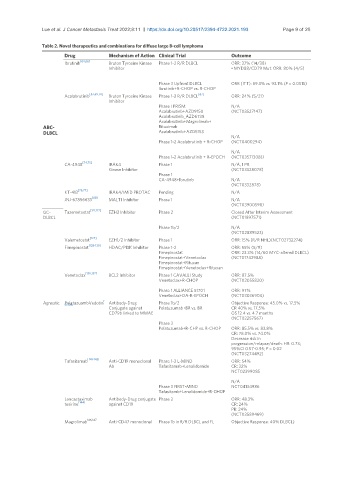
Table 2. Novel therapeutics and combinations for diffuse large B-cell lymphoma
Drug Mechanism of Action Clinical Trial Outcome
Ibrutinib [64,66] Bruton Tyrosine Kinase Phase 1-2 R/R DLBCL ORR: 37% (14/38)
Inhibitor • MYD88/CD79 Mut: ORR: 80% (4/5)
Phase 3 Upfront DLBCL ORR (ITT): 89.3% vs. 93.1% (P = 0.0515)
Ibrutinib+R-CHOP vs. R-CHOP
[67,69,70] [67]
Acalabrutinib Bruton Tyrosine Kinase Phase 1-2 R/R DLBCL ORR: 24% (5/21)
Inhibitor
Phase I PRISM: N/A
Acalabrutinb+AZD9150 (NCT03527147)
Acalabrutinib_AZD6738
Acalabrutinib+Magrolimab+
ABC- Rituximab
DLBCL Acalabrutinib+AZD5153
N/A
Phase 1-2 Acalabrutinib + R-CHOP (NCT0400294)
N/A
Phase 1-2 Acalabrutinib + R-EPOCH (NCT03571308))
[74,75]
CA-4948 IRAK4 Phase 1 N/A, 1 PR
Kinase Inhibitor (NCT03328078)
Phase 1
CA-4948+Ibrutinb N/A
(NCT0332878)
[76,77]
KT-413 IRAK4/IMiD PROTAC Pending N/A
JNJ-67856633 [88] MALT1 Inhibitor Phase 1 N/A
(NCT03900598)
GC- Tazemetostat [121,122] EZH2 Inhibitor Phase 2 Closed After Interim Assessment
DLBCL (NCT01897571)
Phase 1b/2 N/A
(NCT02889523)
[124]
Valemetostat EZH1/2 Inhibitor Phase 1 ORR: 15% (R/R NHL)(NCT02732274)
Fimepinostat [128-130] HDAC/PI3K Inhibitor Phase 1-2 ORR: 55% (5/9)
Fimepinostat ORR: 23.3% (14/60 MYC-altered DLBCL)
Fimepinostat+Venetoclax (NCT01742988)
Fimepinostat+Rituxan
Fimepinostat+Venetoclax+Rituxan
Venetoclax [136,137] BCL2 Inhibitor Phase 1 CAVALLI Study ORR: 87.5%
Venetoclax+R-CHOP (NCT02055820)
Phase 1 ALLIANCE 51701 ORR: 97%
Venetoclax+DA-R-EPOCH (NCT03036904)
[
Agnostic PolatuzuambVedotin Antibody-Drug Phase 1b/2 Objective Response: 45.0% vs. 17.5%
51,139]
Conjugate against Polatuzumab+BR vs. BR CR 40% vs. 17.5%
CD79b linked to MMAE OS 12.4 vs. 4.7 months
(NCT02257567)
Phase 3
Polatuzumab+R-CHP vs. R-CHOP ORR: 85.5% vs. 83.8%
CR: 78.0% vs. 74.0%
Decrease risk in
progression/relapse/death: HR: 0.73;
95%CI 0.57-0.95; P = 0.02
(NCT03274492)
[141-143]
Tafasitamab Anti-CD19 monoclonal Phase 1-2 L-MIND ORR: 54%
Ab Tafasitamab+Lenalidomide CR: 32%
NCT02399085
N/A
Phase 3 FIRST-MIND NCT04134986
Tafasitamb+Lenalidomide+R-CHOP
Loncastuximab Antibody-Drug conjugate Phase 2 ORR: 48.3%
tesirine [144] against CD19 CR: 24%
PR: 24%
(NCT03589469)
146,147
Magrolimab Anti-CD47 monoclonal Phase 1b in R/R DLBCL and FL Objective Response: 40% DLBCL)