Page 90 - Read Online
P. 90
Solimando et al. J Cancer Metastasis Treat 2022;8:9 https://dx.doi.org/10.20517/2394-4722.2021.166 Page 5 of 12
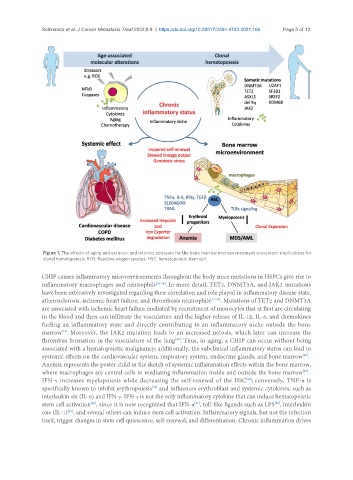
Figure 1. The effects of aging and extrinsic and intrinsic stressors for the bone marrow microenvironment ecosystem: implications for
clonal hematopoiesis. ROS: Reactive oxygen species; HSC: hematopoietic stem cell.
CHIP causes inflammatory microenvironments throughout the body since mutations in HSPCs give rise to
inflammatory macrophages and neutrophils [32-34] . In more detail, TET2, DNMT3A, and JAK2 mutations
have been extensively investigated regarding their correlation and role played in inflammatory disease state,
atherosclerosis, ischemic heart failure, and thrombosis neutrophils [32-34] . Mutations of TET2 and DNMT3A
are associated with ischemic heart failure mediated by recruitment of monocytes that at first are circulating
in the blood and then can infiltrate the vasculature and the higher release of IL-1β, IL-6, and chemokines
fueling an inflammatory state and directly contributing to an inflammatory niche outside the bone
[32]
marrow . Moreover, the JAK2 mutation leads to an increased netosis, which later can increase the
[35]
thrombus formation in the vasculature of the lung . Thus, in aging, a CHIP can occur without being
associated with a hematopoietic malignancy; additionally, the subclinical inflammatory status can lead to
systemic effects on the cardiovascular system, respiratory system, endocrine glands, and bone marrow .
[36]
Anemia represents the poster child in the sketch of systemic inflammation effects within the bone marrow,
where macrophages are central cells in mediating inflammation inside and outside the bone marrow .
[37]
IFN-γ increases myelopoiesis while decreasing the self-renewal of the HSC ; conversely, TNF-α is
[38]
specifically known to inhibit erythropoiesis and influences erythroblast and systemic cytokines, such as
[39]
interleukin six (IL-6) and IFN-γ. IFN-γ is not the only inflammatory cytokine that can induce hematopoietic
[41]
stem cell activation , since it is now recognized that IFN-α , toll-like ligands such as LPS , interleukin
[40]
[42]
one (IL-1) , and several others can induce stem cell activation. Inflammatory signals, but not the infection
[43]
itself, trigger changes in stem cell quiescence, self-renewal, and differentiation. Chronic inflammation drives