Page 74 - Read Online
P. 74
García-Pardo et al. J Cancer Metastasis Treat 2021;7:62 https://dx.doi.org/10.20517/2394-4722.2021.103 Page 11 of 22
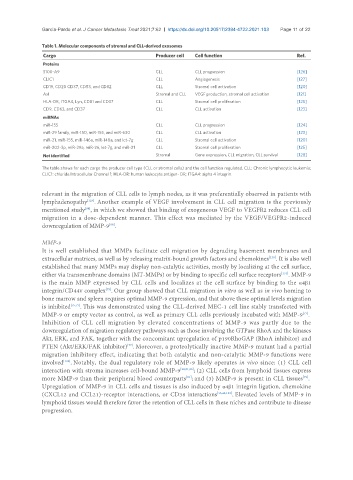
Table 1. Molecular components of stromal and CLL-derived exosomes
Cargo Producer cell Cell function Ref.
Proteins
S100-A9 CLL CLL progression [126]
CLIC1 CLL Angiogenesis [127]
CD19, CD20 CD37, CD53, and CD82 CLL Stromal cell activation [120]
Axl Stromal and CLL VEGF production, stromal cell activation [121]
HLA-DR, ITGA4, Lyn, CD81 and CD37 CLL Stromal cell proliferation [125]
CD9, CD63, and CD37 CLL CLL activation [123]
miRNAs
miR-155 CLL CLL progression [124]
miR-29 family, miR-150, miR-155, and miR-630 CLL CLL activation [123]
miR-21, miR-155, miR-146a, miR-148a, and let-7g CLL Stromal cell activation [120]
miR-202-3p, miR-29a, miR-26, let-7g, and miR-21 CLL Stromal cell proliferation [125]
Not identified Stromal Gene expression, CLL migration, CLL survival [128]
The table shows for each cargo the producer cell type (CLL or stromal cells) and the cell function regulated. CLL: Chronic lymphocytic leukemia;
CLIC1: chloride Intracellular Channel 1; HLA-DR: human leukocyte antigen- DR; ITGA4: alpha 4 integrin.
relevant in the migration of CLL cells to lymph nodes, as it was preferentially observed in patients with
lymphadenopathy . Another example of VEGF involvement in CLL cell migration is the previously
[129]
mentioned study , in which we showed that binding of exogeneous VEGF to VEGFR2 reduces CLL cell
[88]
migration in a dose-dependent manner. This effect was mediated by the VEGF/VEGFR2-induced
downregulation of MMP-9 .
[88]
MMP-9
It is well established that MMPs facilitate cell migration by degrading basement membranes and
[130]
extracellular matrices, as well as by releasing matrix-bound growth factors and chemokines . It is also well
established that many MMPs may display non-catalytic activities, mostly by localizing at the cell surface,
either via transmembrane domains (MT-MMPs) or by binding to specific cell surface receptors . MMP-9
[131]
is the main MMP expressed by CLL cells and localizes at the cell surface by binding to the α4β1
integrin/CD44v complex . Our group showed that CLL migration in vitro as well as in vivo homing to
[75]
bone marrow and spleen requires optimal MMP-9 expression, and that above these optimal levels migration
is inhibited [75,77] . This was demonstrated using the CLL-derived MEC-1 cell line stably transfected with
[77]
MMP-9 or empty vector as control, as well as primary CLL cells previously incubated with MMP-9 .
Inhibition of CLL cell migration by elevated concentrations of MMP-9 was partly due to the
downregulation of migration regulatory pathways such as those involving the GTPase RhoA and the kinases
Akt, ERK, and FAK, together with the concomitant upregulation of p190RhoGAP (RhoA inhibitor) and
PTEN (Akt/ERK/FAK inhibitor) . Moreover, a proteolytically inactive MMP-9 mutant had a partial
[77]
migration inhibitory effect, indicating that both catalytic and non-catalytic MMP-9 functions were
involved . Notably, the dual regulatory role of MMP-9 likely operates in vivo since: (1) CLL cell
[132]
interaction with stroma increases cell-bound MMP-9 [32,81,85] ; (2) CLL cells from lymphoid tissues express
more MMP-9 than their peripheral blood counterparts ; and (3) MMP-9 is present in CLL tissues .
[81]
[79]
Upregulation of MMP-9 in CLL cells and tissues is also induced by α4β1 integrin ligation, chemokine
(CXCL12 and CCL21)-receptor interactions, or CD38 interactions [15,42,133] . Elevated levels of MMP-9 in
lymphoid tissues would therefore favor the retention of CLL cells in these niches and contribute to disease
progression.