Page 60 - Read Online
P. 60
Almeida et al. J Cancer Metastasis Treat 2021;7:57 https://dx.doi.org/10.20517/2394-4722.2021.108 Page 7 of 10
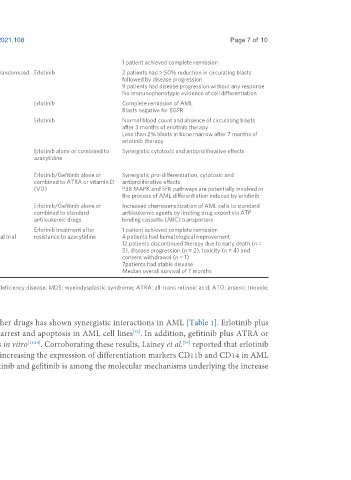
1 patient achieved complete remission
[20]
Sayar et al. , 2015 11 de novo AML patients (2 with relapsed/refractory Pilot prospective non-randomized Erlotinib 2 patients had > 50% reduction in circulating blasts
disease and 9 with previously untreated AML) with a clinical trial followed by disease progression
median age of 76 (range 60-85 years) 9 patients had disease progression without any response
No immunophenotypic evidence of cell differentiation
Chan and 68-year-old male AML patient with concurrent non-small Case report Erlotinib Complete remission of AML
[35]
Pilichowska , 2007 cell lung cancer Blasts negative for EGFR
[52]
Pitini et al. , 2008 64-year-old male AML patient with concurrent non-small Case report Erlotinib Normal blood count and absence of circulating blasts
cell lung cancer after 3 months of erlotinib therapy
Less than 2% blasts in bone marrow after 7 months of
erlotinib therapy
[55]
Lainey et al. , 2013 SKM1, MOLM-13, KG-1, Kasumi-1, HL-60, and MV4-11 In vitro treatment Erlotinib alone or combined to Synergistic cytotoxic and antiproliferative effects
AML cell lines azacytidine
Primary MDS and AML cells
[56]
Lainey et al. , 2013 HL-60 and MOLM-13 AML cell lines In vitro treatment Erlotinib/Gefitinib alone or Synergistic pro-differentiation, cytotoxic and
+
CD34 AML primary blasts combined to ATRA or vitamin D antiproliferative effects
(VD) P38 MAPK and SFK pathways are potentially involved in
the process of AML differentiation induced by erlotinib
[57]
Lainey et al. , 2012 KG-1 AML cells In vitro treatment Erlotinib/Gefitinib alone or Increased chemosensitization of AML cells to standard
+
CD34 AML primary blasts combined to standard antileukemic agents by limiting drug export via ATP
antileukemic drugs binding cassette (ABC) transporters
[58]
Thepot et al. , 2014 30 MDS/AML patients with a median age of 77.5 (range Phase I/II prospective Erlotinib treatment after 1 patient achieved complete remission
53-86 years) non-randomized clinical trial resistance to azacytidine 4 patients had hematological improvement
12 patients discontinued therapy due to early death (n =
5), disease progression (n = 2), toxicity (n = 4) and
consent withdrawal (n = 1)
7patients had stable disease
Median overall survival of 7 months
EGFR: Epidermal growth factor receptor; AML: acute myeloid leukemia; SCID: severe combined immunodeficiency disease; MDS: myelodysplastic syndrome; ATRA: all-trans retinoic acid; ATO: arsenic trioxide;
RNAi: RNA interference.
In addition to the use of EGFR TKI as single agents, the combination with other drugs has shown synergistic interactions in AML [Table 1]. Erlotinib plus
[55]
azacytidine, an inhibitor of DNA methyltransferases, increased the cell cycle arrest and apoptosis in AML cell lines . In addition, gefitinib plus ATRA or
arsenic trioxide potentiated the differentiation of APL and non-APL AML cells in vitro [13,14] . Corroborating these results, Lainey et al. reported that erlotinib
[56]
and gefitinib acted synergistically when associated with ATRA and vitamin D, increasing the expression of differentiation markers CD11b and CD14 in AML
cells. In this context, the inhibition of drug efflux via ABC transporters by erlotinib and gefitinib is among the molecular mechanisms underlying the increase
of AML chemosensitization .
[57]