Page 81 - Read Online
P. 81
Page 4 of 8 Moia et al. J Cancer Metastasis Treat 2019;5:67 I http://dx.doi.org/10.20517/2394-4722.2019.020
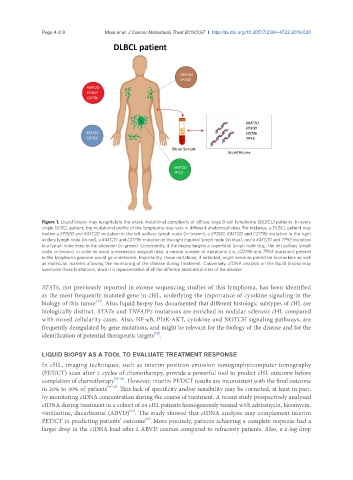
Figure 1. Liquid biopsy may recapitulate the entire mutational complexity of diffuse large B-cell lymphoma (DLBCL) patients. In every
single DLBCL patient, the mutational profile of the lymphoma may vary in different anatomical sites. For instance, a DLBCL patient may
harbor a EP300 and KMT2D mutation in the left axillary lymph node (in brown), a EP300, KMT2D and CD79b mutation in the right
axillary lymph node (in red), a KMT2D and CD79b mutation in the right inguinal lymph node (in blue), and a KMT2D and TP53 mutation
in a lymph node deep in the abdomen (in green). Consistently, if the biopsy targets a superficial lymph node (e.g., the left axillary lymph
node; in brown), in order to avoid unnecessary surgical risks, a certain number of mutations (i.e., CD79b and TP53 mutation) present
in the lymphoma genome would go undetected. Importantly, those mutations, if detected, might serve as predictive biomarkers as well
as molecular markers allowing the monitoring of the disease during treatment. Conversely, cfDNA analysis on the liquid biopsy may
overcome these limitations, since it is representative of all the different anatomical sites of the disease
STAT6, not previously reported in exome sequencing studies of this lymphoma, has been identified
as the most frequently mutated gene in cHL, underlying the importance of cytokine signaling in the
[23]
biology of this tumor . Also, liquid biopsy has documented that different histologic subtypes of cHL are
biologically distinct. STAT6 and TNFAIP3 mutations are enriched in nodular sclerosis cHL compared
with mixed cellularity cases. Also, NF-kB, PI3K-AKT, cytokine and NOTCH signaling pathways, are
frequently deregulated by gene mutations, and might be relevant for the biology of the disease and for the
[23]
identification of potential therapeutic targets .
LIQUID BIOPSY AS A TOOL TO EVALUATE TREATMENT RESPONSE
In cHL, imaging techniques, such as interim positron emission tomography/computer tomography
(PET/CT) scan after 2 cycles of chemotherapy, provide a powerful tool to predict cHL outcome before
completion of chemotherapy [24-26] . However, interim PET/CT results are inconsistent with the final outcome
in 20% to 30% of patients [24-26] . This lack of specificity and/or sensibility may be corrected, at least in part,
by monitoring ctDNA concentration during the course of treatment. A recent study prospectively analyzed
ctDNA during treatment in a cohort of 24 cHL patients homogenously treated with adriamycin, bleomycin,
[23]
vinblastine, dacarbazine (ABVD) . The study showed that ctDNA analysis may complement interim
[23]
PET/CT in predicting patients’ outcome . More precisely, patients achieving a complete response had a
larger drop in the ctDNA load after 2 ABVD courses compared to refractory patients. Also, a 2-log drop